
EffiChem is a leader in producing effective and efficient laboratory quality solutions. We’ve been providing our customers with easy-to-use, highly configurable method validation and laboratory information and quality management software for more than 15 years.
Founded in 1999, EffiChem has been providing laboratory quality professionals with the tools they need to accurately and efficiently validate testing methods and manage quality and laboratory information for more than 15 years. We have been expanding ever since.
EffiChem is now used by more than 550 institutions in more than 30 countries worldwide.
EffiChem exists to provide its users with confidence in quality. We believe that quality means not just helping customers achieve their compliance goals, but also in doing so with products that reduce time and stress, and which increase efficiency within the lab.
We work with our customers to help them lower their total cost of quality by giving them not just the solutions they need to be effective in their work and respond to inspections but also to identify possible quality-related issues in advance and identify areas for improvement.
EffiChem’s products are designed to make this process even more effective. Not only are we the only provider in the industry to combine method validation, laboratory information and quality management with CFR Part 11 recordkeeping. EffiChem is designed to be easy to use with features that constantly improved to provide greater flexibility and power to meet your laboratory’s needs – whether they be in method validation, quality/information management, or a combination of the two.
Try EffiChem free for 30 days. 5 minute install.
Switch to EffiChem
EffiChem gives you confidence, not only in quality laboratory results and products, but in the data that you provide to auditors, accreditation, and inspectors.
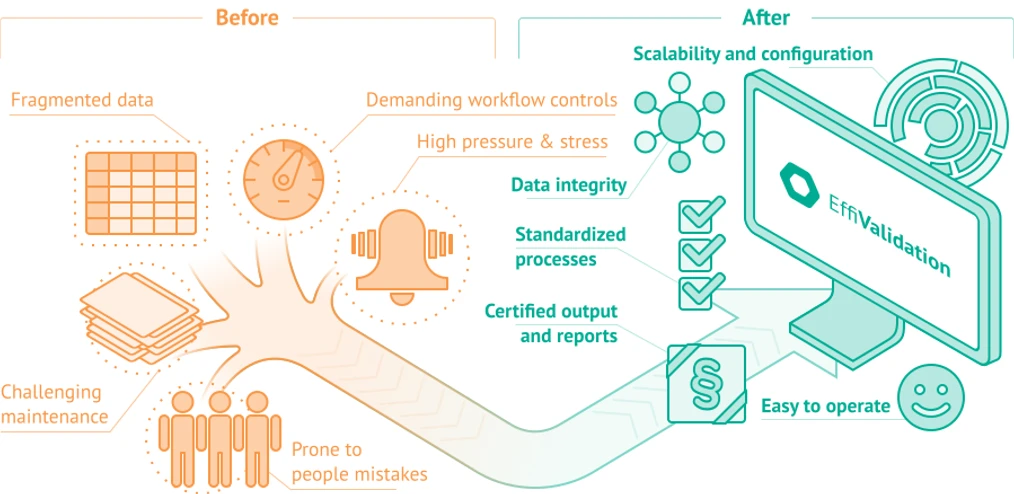 Effichem: Switch to EffiChem and Complicated Processes Become Simple
Effichem: Switch to EffiChem and Complicated Processes Become Simple
GAMP compliance
EffiChem software is designed and developed in accordance with GAMP 5 guidance. EffiChem ensures the proper documentation and execution of all validation activities, specifically the Specifications and Testing components of GAMP 5 so that your lab’s computer systems create as little risk as possible.
FDA 21 CFR Part 11 Compliance
EffiChem software is a closed system, with all 21 CFR Part 11 requirements accounted for, including limited system access, electronic audit trail, accurate copies, and accurate retrieval. You stay compliant with all current industry trends focused on data integrity and traceability.
ISO 17025 Technical Requirements
EffiValidation was designed to not only reduce time, but improve quality and efficiency by taking the ambiguity out of method validation, while ensuring that data integrity is protected, and results are available at the click of a mouse. This means that you can save up to 60% more time when using EffiValidation.
ISO 17025 Management Requirements
EffiQS covers the whole laboratory process, enabling you to manage every relevant aspect of your quality process from one place, and keep authenticated records that help you maintain ISO 17025 accreditation, GMP standards, and 21 CFR Part 11 compliant records.