Scoring of LC separation procedures for ezetimibe and its degradants using Mgears Chrom Best Method

Mestrelab Research: Scoring of LC separation procedures for ezetimibe and its degradants using Mgears Chrom Best Method
In industries with discovery departments and purification processes, preparative chromatography is key for obtaining high-purity materials. But screening sample sets and analyzing results manually can be time-consuming. In this application note, Prof. Rafael Cela, Senior Researcher at the Mestrelab Research Center (CIM), showcases the selection of LC procedures using Mgears Chrom Best Method and highlights how automation streamlines the screening process and helps in analysis, reporting, and decision-making.
Selected sections from the application note follows.
Screening sample sets are common tasks in industries with discovery departments, and purification and scaling fabrication processes for high added value chemicals. In all these fields, preparative chromatography is often the preferred way to obtain material with sufficient purity. The efficient purification methodology involves screening a few chromatography methods using mass and/or UV detectors and testing different columns and/or mobile phase compositions.
Frequently, these sample sets consider chiral compounds, and the objective is not only the separation of the target species such that it is as pure as possible, but also the separation of enantiomers and/or diasteroisomeric impurities. From this screen, critical decisions are taken (e.g., a synthetic route is preferred; a preparative method is chosen; alternative separation methods are tested; ...). Frequently, a busy discovery and/or purification lab receives many such requests and reviewing the results of screens manually is time-consuming and tedious. A tool which automates the task, gathering the required data, scoring the methods for suitability, and reporting the results, is therefore highly desirable.
Mgears Chrom Best Method has been developed as a tool to hierarchically organize the results of screening experiments according to flexible, convenient, automated, and user-selectable scoring criteria. In this short note, we will consider, as a case study, the selection of LC procedures to isolate ezetimibe (C24 H21 F2NO3; CAS: 163222-33-1, MW: 409.433 g/mol) from several of its degradants.
Degradation processes of ezetimibe have been extensively described in the literature1,2 . The sample mixture was prepared departing from an ezetimibe standard (Acesys Pharmatech, Cat No. 1242-111-21, Fairfield, NJ, USA). This standard was submitted to alkaline and acidic degradation processes, then mixed and spiked with the pure standard to provide a mixture where both the API and several of its degradants can be accurately detected. This mixture was diluted and used for all screening runs described, which were arranged according to a simple factorial experimental design (Table 1).
The column was a Cortecs C8 3 x 50 mm, 2.7 um (Waters® PN.186008369). The instrumental system used was a Waters® Acquity HClass LC system furnished with an Acquity DAD-UV detector and a Xevo TQD detector and was operated via the Masslynx software. MSD scan mode signal in the 100-700 m/z range was registered in negative polarity. The DAD wavelength range was 220-400 nm. The mobile phase flowrate was 0.2 mL/min in all experiments. The pH of the mobile phases was varied between 3 and 8, developing elutions with acetonitrile as modifier, at column temperatures of between 35 and 55 ºC.
Chrom Best Method Scoring Criteria
Chrom Best Method provides a highly flexible scoring system that can be adapted to the needs of any chromatographic separation aimed at selecting the optimal purification method. Factors such as the target resolution, purity, symmetry, and retention time can be evaluated by means of desirability functions and combined into an overall measure, allowing the selection of the best separation among those provided in the experiment.
Desirability functions for scoring criteria use logistic functions (Figure 1) that represent situations that range from highly undesirable (score = 0) to highly desirable (score = 1). These functions are defined by assigning a value for the 50% score point and a slope (k). Moreover, a weight coefficient can be assigned to reflect the relative importance of each scoring criterion to the user.
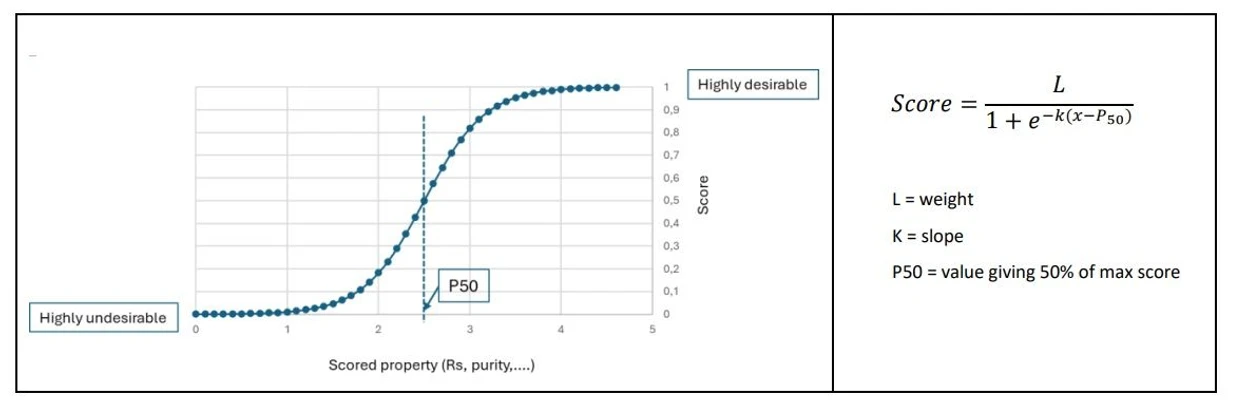 Mestrelab Research: Figure 1. Desirability functions used by Chrom Best Method
Mestrelab Research: Figure 1. Desirability functions used by Chrom Best Method
Some of these functions are quite straightforward, such as those for peak purity, symmetry, and retention time. For example, the purity criterion is defined by fixing the purity level for P50 (e.g., 95%) and the slope. This allows for more or less demanding conditions for separation. For example, in Figure 2 we see how the purity criterion can be relaxed, changing the slope value even though the P50 condition remains the same. Similar behavior may be found in other unilateral desirability criteria (e.g., resolution and similarity), and even in the retention time criterion, although in this case the desirability function defined is a bilateral one.
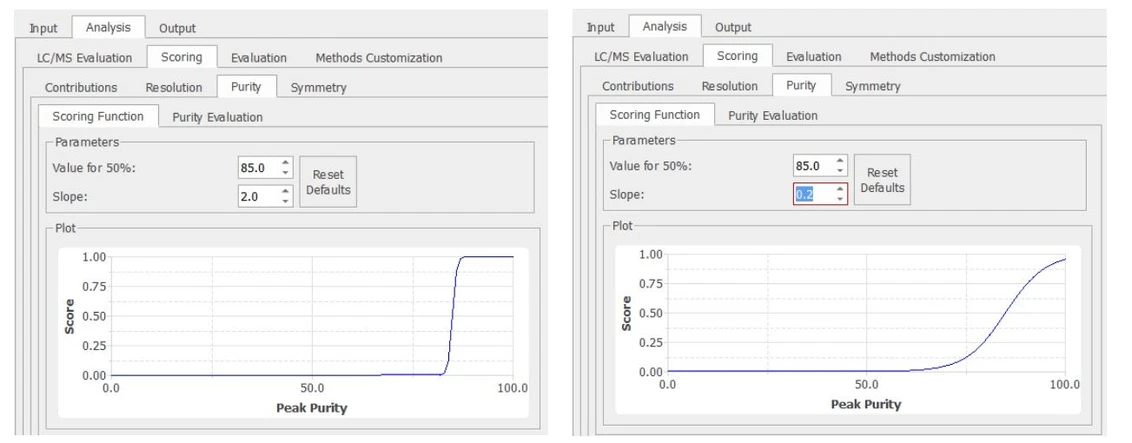 Mestrelab Research - Figure 2. Controlling the target peak purity criterion.jpg
Mestrelab Research - Figure 2. Controlling the target peak purity criterion.jpg
Resolution criterion is a bit more complex because, to allow maximal flexibility, critical values are defined for peaks eluting before and/or after the target peak. Moreover, the basic Rs criterion is combined with another term that considers the maximum percentage of impurity allowable. In this way, the criterion can be controlled and adapted to virtually any practical situation.
...
1Stability indicating RP-HPLC method for simultaneous determination of simvastatin and ezetimibe from tablet dosage form, R.P. Dixit, C.R. Barhate, S.G. Padhye, C.L. Viswanathan, M.S. Nagarsenker, Ind. Jour. Pham. Sci. 2010, 72(2) 204-210.
2Development and validation of a novel stability indicating HPLC method for the quantitative determination of eleven related substances in ezetimibe drug substance and drug product. Zhiqiang Luo, Zhongqing Deng, Yang Liu, Guopeng Wang, Wenning Yang, Chengbo Hou, Minming Tang, Riurui Yang, Huaming Zhou, Talanta, 2015, 139, 67-74.