Research from IOCB Prague reveals a previously unknown mechanism of genetic transcription

- Photo: IOCB Prague: Research from IOCB Prague reveals a previously unknown mechanism of genetic transcription
- Video: IOCB Prague: Research from IOCB Prague reveals a previously unknown mechanism of genetic transcription
Scientists at IOCB Prague are uncovering new details of gene transcription. They have identified a previously unknown molecular mechanism by which the transcription of genetic information from deoxyribonucleic acid (DNA) into ribonucleic acid (RNA) can be initiated. The researchers focused on a specific class of molecules known as alarmones, which are found in cells across a wide range of organisms and whose levels often increase under conditions of cellular stress. The results were published in the prestigious scientific journal Nature Chemical Biology.
 IOCB Prague/Tomáš Belloň: Research from IOCB Prague reveals a previously unknown mechanism of genetic transcription: Dr. Hana Cahová, head of the Chemical Biology of Nucleic Acids research group.
IOCB Prague/Tomáš Belloň: Research from IOCB Prague reveals a previously unknown mechanism of genetic transcription: Dr. Hana Cahová, head of the Chemical Biology of Nucleic Acids research group.
Molecules of ribonucleic acid can carry a variety of chemical modifications at one end, known as caps. In eukaryotic organisms, including human cells, the best-known cap plays an important role in RNA stability and in regulating its subsequent fate. In recent years, however, it has become clear that alternative, non-canonical RNA caps also exist, although their formation and the mechanisms by which they are attached to RNA remain only partially understood. These include alarmone caps, formed by dinucleoside polyphosphate molecules, that protect cellular RNA at moments when the cell is under threat.
In their study, Dr. Hana Cahová and colleagues examined bacterial RNA polymerase and investigated how this enzyme can initiate transcription using dinucleoside polyphosphates (NpNs) instead of the standard RNA building blocks. For the first time, the scientists described, at the atomic level, how RNA bearing an alarmone cap can be generated directly at the initiation of gene transcription. They also observed that NpNs bind through a different type of base pairing than is typically observed.
The work was significantly shaped by Valentina Serianni from Hana Cahová’s team, who demonstrated the ability of dinucleoside polyphosphates to initiate gene transcription, and by Jana Škerlová, who focused on the structural analysis of RNA polymerase. Using cryogenic electron microscopy data, she showed how dinucleoside polyphosphate molecules bind within the active site of RNA polymerase – that is, the core of the enzyme where genetic information is transcribed. Together, their findings help to elucidate the processes that accompany the transcription of genetic information from DNA to RNA.
Hana Cahová adds: “We’re describing something that truly occurs in cells and that we’re now able to observe directly at the level of individual molecules. This allows us to answer fundamental questions about cellular processes, such as how cells adapt to stress. RNA plays a central role in this, as it carries the cascade of information underlying any cellular response – for example, to threatening conditions caused by nutrient deprivation or temperature shock.”
 IOCB Prague/Tomáš Belloň: Research from IOCB Prague reveals a previously unknown mechanism of genetic transcription: Dr. Tomáš Kouba, head of the Cryogenic Electron Microscopy core facility.
IOCB Prague/Tomáš Belloň: Research from IOCB Prague reveals a previously unknown mechanism of genetic transcription: Dr. Tomáš Kouba, head of the Cryogenic Electron Microscopy core facility.
Cryogenic electron microscopy (cryo-EM) was central to the project. This part of the work was led by Dr. Tomáš Kouba, one of the authors of the study. “Cryogenic electron microscopy allows us to freeze biological molecules in a state very close to their natural form and then determine their three-dimensional structure. This makes it possible to look directly into the active centers of enzymes and observe their function down to the atomic level,” he explains.
Last year, IOCB Prague opened a new cryo-EM center that is unique within the Czech research environment. State-of-the-art cryogenic electron microscopes are housed in a specially designed building and enable the study of biological processes with exceptional structural precision.
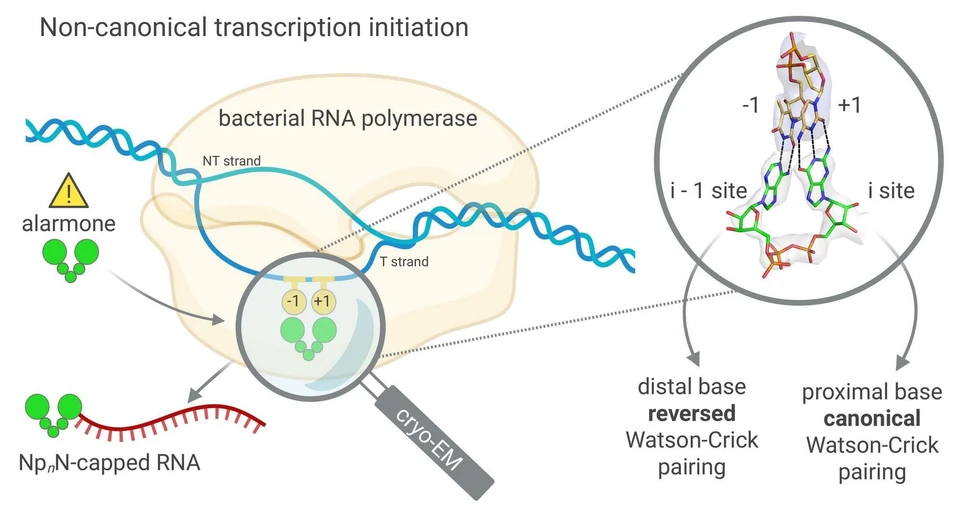 IOCB Prague: Research from IOCB Prague reveals a previously unknown mechanism of genetic transcription: This work shows how NpnNs initiate bacterial transcription. Cryo-EM reveals how nucleobases pair with the template: one in canonical and one in reverse Watson–Crick mode, providing a structural basis for their function as non-canonical RNA caps.
IOCB Prague: Research from IOCB Prague reveals a previously unknown mechanism of genetic transcription: This work shows how NpnNs initiate bacterial transcription. Cryo-EM reveals how nucleobases pair with the template: one in canonical and one in reverse Watson–Crick mode, providing a structural basis for their function as non-canonical RNA caps.
The Original article
Molecular insight into 5′ RNA capping with NpnNs by bacterial RNA polymerase
Valentina M. Serianni, Jana Škerlová, Anna Knopp Dubánková, Anton Škríba, Hana Šváchová, Tereza Vučková, Anatolij Filimoněnko, Milan Fábry, Pavlína Řezáčová, Tomáš Kouba & Hana Cahova
Nat. Chem. Biol. 21 (2026)
https://doi.org/10.1038/s41589-025-02134-5
licensed under CC-BY 4.0
Abstract
RNA capped with dinucleoside polyphosphates has been discovered in bacteria and eukaryotes only recently. The likely mechanism of this specific capping involves direct incorporation of dinucleoside polyphosphates by RNA polymerase as noncanonical initiating nucleotides. However, how these compounds bind into the active site of RNA polymerase during transcription initiation is unknown. Here, we explored transcription initiation in vitro, using a series of DNA templates in combination with dinucleoside polyphosphates and model RNA polymerase from Thermus thermophilus. We observed that the transcription start site can vary on the basis of the compatibility of the specific template and dinucleoside polyphosphate. Cryo-electron microscopy structures of transcription initiation complexes with dinucleoside polyphosphates revealed that both nucleobase moieties can pair with the DNA template. The first encoded nucleotide pairs in a canonical Watson–Crick manner, whereas the second nucleobase pairs noncanonically in a reverse Watson–Crick manner. Our work provides a structural explanation of how dinucleoside polyphosphates initiate RNA transcription.