
ISTRAN EcaLayer Metal Layer Analyser
The coulometric technique is used for measuring the thickness of electroplated deposits on a wide variety of substrates such as steel, aluminium, copper, nickel, silver, alloys, some insulators.
The coulometric method ensures accurate and precise results. The analyser is extremely simple to use and therefore it is also suitable for routine measurements not needing any special qualification. Moreover, coulometry is the only method allowing the measurement of multi-layer coatings such as chrome, chrome oxide on tin, tin, tin-iron intermetals on steel, etc.
Measuring principle
The fundamental principle is the Faraday’s Law. The method is similar to the electrolytic plating process but here the coating is removed electrochemically from a defined surface area. For this purpose, a piece of the sample is placed to the flow-through measuring cell which is filled with a suitable electrolyte solution and the analysed metal coating is subsequently removed by applying an appropriate current. Hence, metal layers are measured by anodic dissolution, metal oxide layers, such as tin oxide on a tin coating, are measured by cathodic dissolution. The measured sample area is given by the gasket used and it can include 0.2 to 20 cm2. The analysis is performed totally automatically and is controlled by a PC.
By applying the Faraday’s Laws, the thickness of a metal layer can be calculated.
The EcaLayer Model 220/220a consists of three major parts:
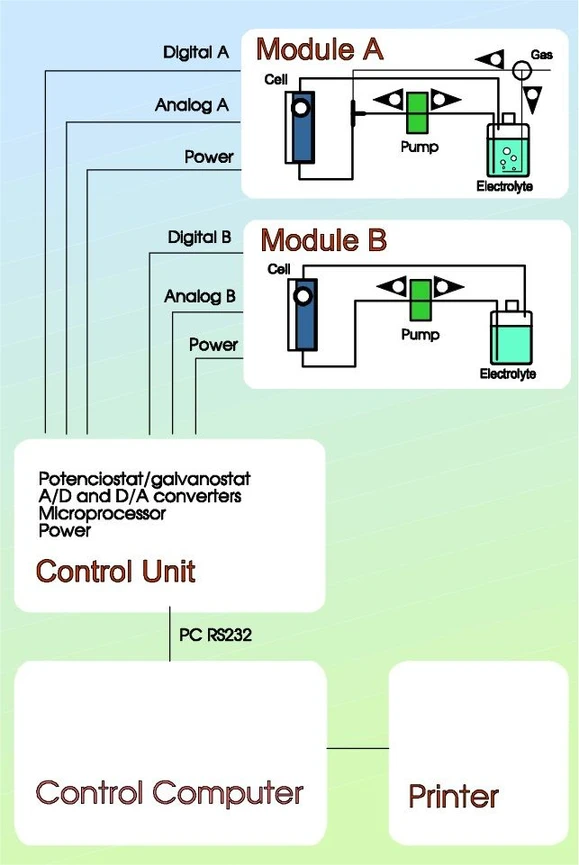 ISTRAN EcaLayer Metal Layer Analyser - Scheme
ISTRAN EcaLayer Metal Layer Analyser - Scheme1. Analytical Unit
Two types of analytical units can be used:
- Module A for metal coatings and metal oxide layer measurements needing the removal of dissolved oxygen, e.g. tin oxide measurements, and
- Module B for measurements not needing oxygen removal, e.g. tin layer thickness measurements.
Module A encloses a unique flow-through analytical cell with the sample as the working electrode, a stainless steel counter and Ag/AgCl reference electrodes, a peristaltic pump, an electromagnetic valve for the gas and a reservoir for the electrolyte solution.
Module B is built in the same way but without the gas unit.
2. Control Unit
The control unit comprises a computer controlled potentiostat/galvanostat with a maximum output of 100 mA, 16-bit A/D and D/A converters. The unit is controlled by a microprocessor linked to the PC through a serial line.
3. Computer and software
Any IBM compatible PC can be coupled to the instrument. The user-friendly software facilitates the use without any special knowledge about electrochermistry. GLP facilities such as archiving, printing of reports, selftesting are included.
Function
The metal layer analyser works as follows:
- The sample is placed into the cell and the cell is closed. The next steps are performed automatically by computer control.
- The cell is filled with the electrolyte from the reservoir. If needed, the electrolyte solution is with an inert gas to remove dissolved oxygen.
- The coating is removed by applying a suitable constant current and the potential of the sample material (i.e. the working electrode) is monitored and registered in the time.
- The electrolyte solution is removed from the cell.
- In the meantime, the registered potential-time dependence is evaluated giving the dissolution time and the layer thickness is calculated automatically.
- The cell is opened and the sample is removed. The whole procedure takes 4 to 8 minutes.
Some applications
Measurements of tin coatings on steel
Tin coatings are measured by anodic dissolution in HCl electrolyte solution with currents up to 100 mA. Both the tin and the tin-iron intermetallic layers can be measured in a single run.
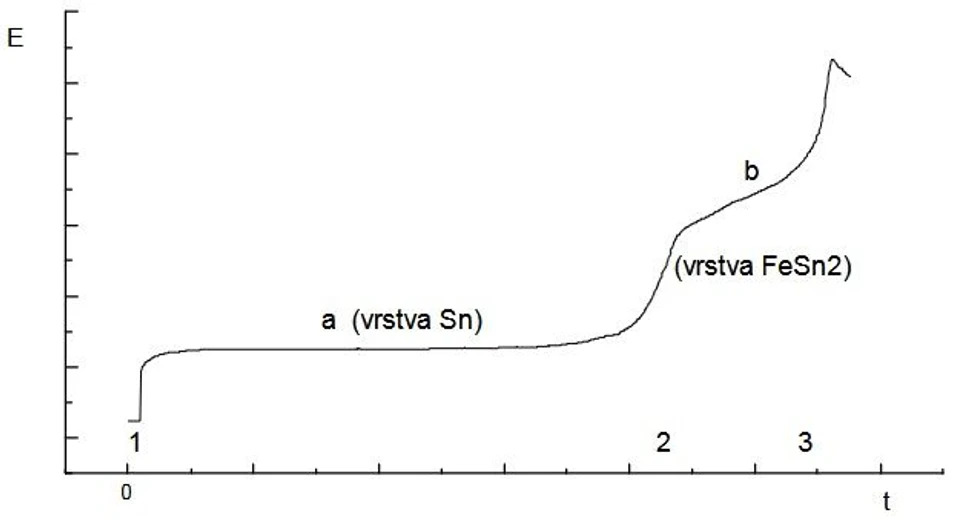 ISTRAN EcaLayer Metal Layer Analyser: Measurements of tin coatings on steel
ISTRAN EcaLayer Metal Layer Analyser: Measurements of tin coatings on steelMeasurement of passivating chromous layers on tin coated steel
The chromium layers are dissolved by anodic oxidation in a phosphate buffer electrolyte solution with currents of few mA.
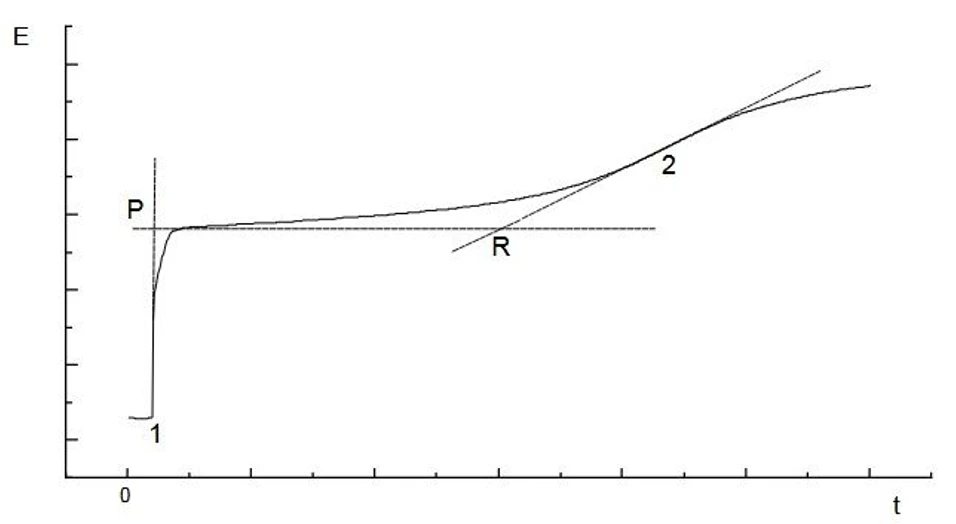 ISTRAN EcaLayer Metal Layer Analyser: Measurement of passivating chromous layers on tin coated steel
ISTRAN EcaLayer Metal Layer Analyser: Measurement of passivating chromous layers on tin coated steelMeasurement of oxide layers on tin coatings
Oxide layers can be determined by cathodic dissolution in deairated HBr electrolyte solution with currents about -1 mA. Due to the uncertain stoichiometry of the oxide layers, the results are expressed as charge consumed per sample area.
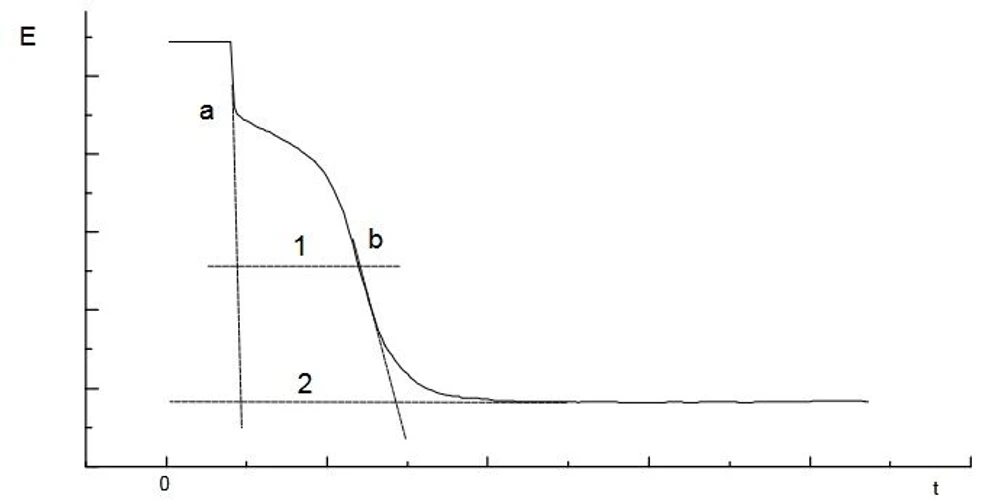 ISTRAN EcaLayer Metal Layer Analyser: Measurement of oxide layers on tin coatings
ISTRAN EcaLayer Metal Layer Analyser: Measurement of oxide layers on tin coatingsOrdering information
EcaLayer Model 220 includes
- Control Unit
- Flow System with Module A
- Application software
- PC with printer (specified by the customer)
- All necessary cables
- Users manual
- Methodologies according to EN ISO 2177
EcaLayer Model 220a includes
- Control Unit
- Flow System with Module B
- Application software
- PC with printer (specified by the customer)
- All necessary cables
- Users manual
- Methodologies according to EN ISO 2177
