High-Throughput Chromatographic Separation of Oligonucleotides: A Proof of Concept Using Ultra-Short Columns
- Photo: Anal. Chem. 2023, 95, 27, 10448-10456 - cover image
In the research article published in ACS Analytical Chemistry journal the researchers from the University of Geneva, Switzerland, and Waters Corporation took a deep dive into understanding the retention mechanism of oligonucleotides (ONs), evaluation of the applicability of the linear solvent strength (LSS) retention model, and exploration of the potential of ultra-short columns.
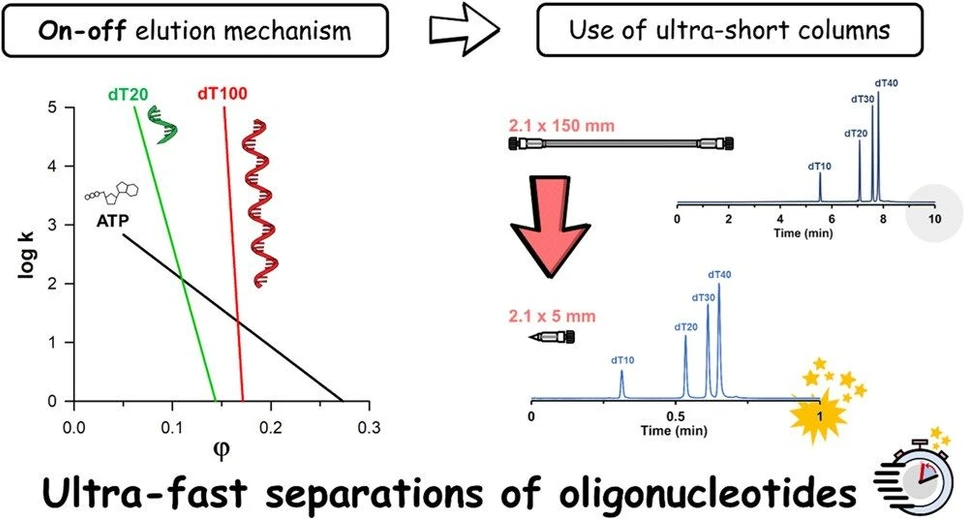
Ion-pairing reversed-phase liquid chromatography (IP-RPLC) serves as a key method for characterizing oligonucleotides and their impurities. This study delved into understanding the retention mechanisms of oligonucleotides, validating the linear solvent strength (LSS) retention model for molecules ranging from 3 to 30 kDa, and confirmed the model's ability to predict retention times accurately. It introduced the use of ultra-short 5 mm columns which facilitated rapid separations of oligonucleotide mixtures in just 30 seconds without compromising separation efficiency. Despite oligonucleotides displaying "on-off" elution patterns similar to smaller proteins, the effectiveness of these shorter columns suggests no further gains in selectivity or efficiency from using longer columns. This finding sets the stage for future research on more complex oligonucleotide formulations using this accelerated method.
The original article
High-Throughput Chromatographic Separation of Oligonucleotides: A Proof of Concept Using Ultra-Short Columns
Honorine Lardeux, Szabolcs Fekete, Matthew Lauber, Valentina D’Atri, and Davy Guillarme
Analytical Chemistry 2023 95 (27), 10448-10456
DOI: 10.1021/acs.analchem.3c01934
licensed under CC-BY 4.0
Selected sections from the article follow.
Abstract
Ion-pairing reversed-phase liquid chromatography (IP-RPLC) is the reference separation technique for characterizing oligonucleotides (ONs) and their related impurities. The aim of this study was to better understand the retention mechanism of ONs, evaluate the applicability of the linear solvent strength (LSS) retention model, and explore the potential of ultra-short columns having a length of only 5 mm for the separation of model ONs. First, the validity of the LSS model was evaluated for ONs having sizes comprised between 3 and 30 kDa, and the accuracy of retention time predictions was assessed. It was found that ONs in IP-RPLC conditions follow an “on–off” elution behavior, despite a molecular weight lower than that of proteins. For most linear gradient separation conditions, a column length between 5 and 35 mm was found to be appropriate. Ultra-short columns of only 5 mm were therefore explored to speed up separations by considering the impact of the instrumentation on the efficiency. Interestingly, the impacts of injection volume and post-column connection tubing on peak capacity were found to be negligible. Finally, it was demonstrated that longer columns would not improve selectivity or separation efficiency, but baseline separation of three model ONs mixtures was enabled in as little as 30 s on the 5 mm column. This proof-of-concept work paves the way for future investigations using more complex therapeutic ONs and their related impurities.
...
Experimental Section
Chemicals and Samples
Water was obtained from a Milli-Q water purification system from Millipore (Bedford, MA). LC-MS grade methanol (MeOH) was purchased from Thermo Fisher Scientific (Reinach, Switzerland). Oligonucleotides were purchased from Eurogentec (Seraing, Switzerland) and Integrated DNA Technologies (IDT, Leuven, Belgium). 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP, ≥99%), triethylamine (TEA, ≥99.5%), and RNase-free water were purchased from Sigma-Aldrich (Buchs, Switzerland).
Sample and Mobile Phase Preparation
100 μM oligonucleotide aliquots were initially prepared by reconstituting lyophilized material in the appropriate volume of RNase-free water and stored at −20 °C.
Equimolar oligonucleotide mixtures were prepared by mixing aliquots and diluting the products to 5 μM in RNase-free water prior to the IP-RPLC analysis (if not stated otherwise). Sample mixtures used to illustrate the retention of oligonucleotides were (first part of the study) the following: dT10–40 (a mixture of seven poly-deoxythymidylic (dT) acids of 10-, 15-, 20-, 25-, 30-, 35-, and 40-mer) and dT40–100 (a mixture of four poly-dT acids of 40-, 60-, 80-, and 100-mer). For the second part of the study, dT40–100, 4 dT (a mixture of four poly-dT acids of 10-, 20-, 30-, and 40-mer), and 4 PS (a mixture of four 20-mer poly-dT acids with 0, 6, 9, and 19 phosphorothioate modifications that replace the phosphodiester linkages) were used.
Mobile phase A was 14 mM TEA in water, containing 100, 160, or 400 mM HFIP, pH 8.2, 8.1, or 7.8, respectively. Mobile phase B was a mixture of 50:50 mobile phase A and methanol.
Chromatographic System, Columns, and Software
All chromatographic separations were performed on a Waters ACQUITY UPLC I-Class System (Milford, MA) equipped with a binary solvent delivery pump, an autosampler with a flow-through-needle (FTN), and a UV detector with a 0.5 μL UV flow cell. The overall extra-column volume was measured as 5.6 μL from the injection seat of the autosampler to the detector cell, while the offset time was equal to 0.87 s. These values were found after plotting system residence time vs reciprocal flow rate (1/F) for various experiments conducted at several flow rates in the absence of a column (zero-dead volume union). Waters ACQUITY UPLC BEH C18 1.7 μm Column, 5 mm × 2.1 mm, 130 Å VanGuard Pre-column was used in this study. Commercial 50 mm and 150 mm × 2.1 mm columns (Waters ACQUITY Premier Oligonucleotide BEH C18 1.7 μm, 130 Å Column) were also used. Data acquisition and instrument control were performed by Empower 3 Software (Waters). The freely available Excel Spreadsheet developed by Guillarme et al. was used to derive LSS parameters and predict retention times. (23,24)
Apparatus and Methodology
Sample volumes of 1 μL were injected using linear gradients (for all separations). The column temperature was set at 60 °C unless stated otherwise. Various semi-empirical models are used in LC to describe the relationship between experimentally observed retention times and mobile phase composition or gradient conditions. In this study, the following model was considered...
Results and Discussion
Retention Properties of Oligonucleotides
Several studies have reported the elution behavior of ONs in IP-RPLC in the presence of various ion-pairing reagents and using different stationary phases. (25−27) Efforts were mainly focused on (1) improving the chromatographic resolution (selectivity and peak capacity) of ONs or (2) improving the LC-MS sensitivity. (25) Relative retention indices and retention time calibration of homo-oligonucleotides in IP-RPLC have been published, (28) but absolute dimensionless measures of retention (like the S parameter of the LSS model) have not been reported yet, at least to the best of our knowledge. In this study, we focus our attention especially on the sensitivity of solute retention to mobile phase composition (S parameter), as this determines the required (effective) column length. We also discuss the log k0 parameter that corresponds to the extrapolated value of k for φ = 0. Here, the change in mobile phase composition was approximated by the change of organic modifier composition (methanol), while neglecting the effect of the small change in additive concentration. (29) It has also been reported that IP-RPLC separations of ONs benefit from shallow gradients, as they elute in relatively sharp peaks, despite the shallow gradient applied. (25) This may indicate that the retention of the solute is very sensitive to the eluent composition (on–off-like behavior). In practice, 50–150 mm long columns are often used for the IP-RPLC analysis of ONs, which is not necessary if the on–off behavior is valid.
...
Illustration of the On–Off Mechanism of Oligonucleotides
In general, IP-RPLC acts as a mixed-mode chromatographic mode combining hydrophobic and electrostatic (ionic) retention mechanisms. However, if the ion-pairing reagent concentration is sufficiently high, elution is mainly driven by hydrophobic interactions occurring between the stationary phase ligands and the hydrophobic part (i.e., alkyl chain) of the ion-pairing reagent molecules.
Figure 2A shows plots of the log retention factor as a function of mobile phase composition (φ) for ATP, dT20, dT100, and a monoclonal antibody (mAb) as a reference. The figure was constructed on the basis of data obtained with the 100 mM HFIP mobile phase, resulting in the highest S values. It clearly illustrates that dT20 already shows an on–off behavior (very steep curve, S = 60). For the dT100, we observed a 2.5 times higher S value (S = 264) compared with an intact mAb (S typically ranges between 100 and 150). The higher S values observed with ONs are probably due to the differences in molecular shape and specific surface available for interaction with the stationary phase (helical straight shape versus Y-shape).
 Analytical Chemistry 2023, 95 (27), 10448-10456 - Figure 2. Logarithmic solute retention factor (A) and relative migration velocity (u/u0) (B) as a function of the volume fraction of the organic mobile phase φ for ATP (black, MW = 0.5 kDa; S = 13), dT20 (green, MW = 6 kDa; S = 60), dT100 (red, MW = 30 kDa; S = 264), and an intact mAb (blue, MW = 150 kDa, S = ∼116).
Analytical Chemistry 2023, 95 (27), 10448-10456 - Figure 2. Logarithmic solute retention factor (A) and relative migration velocity (u/u0) (B) as a function of the volume fraction of the organic mobile phase φ for ATP (black, MW = 0.5 kDa; S = 13), dT20 (green, MW = 6 kDa; S = 60), dT100 (red, MW = 30 kDa; S = 264), and an intact mAb (blue, MW = 150 kDa, S = ∼116).
Figure 2B shows the relative migration velocity as a function of mobile phase composition. Such a plot illustrates the transition between fully adsorbed (“on”) and fully released (“off”) states. The value of S = 264 (and the log k0 = 45.2) means that up to a mobile phase composition of φ = 0.164, the migration velocity of the dT100 is practically zero (bound at the column inlet, its velocity is less than 1% of the interstitial mobile phase velocity), whereas from φ = 0.179, it moves at a velocity greater than 99% of the mobile phase velocity, indicating that the solute is fully desorbed from the column. The transition range between the adsorbed and desorbed states therefore corresponds to a Δφ range of only 1.5% (0.179–0.164). The transition range for the dT20 is somewhat wider, namely, 6.5% of Δφ. This transition typically occurs between 3 and 4% Δφ for an intact mAb in RPLC mode. For the small ATP molecule, we observed a transition range of 31.4% Δφ (from φ = 0.116–0.430).
Figure 3 shows a plot of the column length (Leff) required to effectively retain dT20 and dT100 (at least at ke = 0.5, which is a common practice for small molecule separations) as a function of gradient time and flow rate.
 Analytical Chemistry 2023, 95 (27), 10448-10456 - Figure 3. Color-coded surface plot of the column length (Leff) required to retain dT20 and dT100 with ke = 0.5, as a function of gradient time and flow rate (dc = 2.1 mm ID, ε = 0.68, and Δφ = 0.2).
Analytical Chemistry 2023, 95 (27), 10448-10456 - Figure 3. Color-coded surface plot of the column length (Leff) required to retain dT20 and dT100 with ke = 0.5, as a function of gradient time and flow rate (dc = 2.1 mm ID, ε = 0.68, and Δφ = 0.2).
...
Conclusions
The goal of this proof-of-concept work was to evaluate if ultra-short columns can be used in IP-RPLC for the analysis of model oligonucleotides in gradient elution mode.
First, the validity of the LSS model was evaluated, and the accuracy of retention time predictions was assessed. It was found that ONs in IP-RPLC conditions follow the on–off elution behavior and that for most linear gradient separation conditions an effective column length between 5 and 35 mm was sufficient to obtain the best selectivity and separation efficiency. Therefore, the applicability of ultra-short columns was explored to speed up ON separations. In this regard, the extra-column band broadening induced by the instrumentation was carefully evaluated. Interestingly, the impact of injection volume and post-column connection tubing on peak capacity were found to be negligible. Next, the impact of column length on apparent efficiency was also monitored. First, column length was varied (150, 50, and 5 mm), while flow rate and gradient time were kept constant. Then, various gradient times and flow rates were tested on the three column lengths. In all cases, the on–off retention mechanism of ONs was confirmed. In addition, 5 mm column proved to be an advantageous choice to (i) increase the overall resolution achievable in gradient mode and (ii) perform ultra-fast separations with reasonable kinetic performance.
Optimized ultra-fast separations were therefore developed on the 5 mm column by using a flow rate of 1 mL/min and a gradient time of 1 min. Three model oligonucleotide mixtures including small, large, and PS-modified ONs were analyzed, and a remarkable resolution was especially obtained for the separation of the small ONs mixture. The separations were subsequently pushed to their limits at a higher flow rate (1.75 mL/min) and temperature (90 °C against the previous 60 °C), enabling baseline separation of all of the mixtures in as little as 30 s.
For the first time, a comprehensive study on the demonstration of the applicability of the LSS model to model oligonucleotides’ IP-RPLC separations in gradient elution mode was performed. The understanding of the ONs retention behavior enabled the evaluation of ultra-short columns and the selection of a 5 mm column to boost the ONs baseline separations in less than 1 min. These findings represent the first proof of concept for achieving ONs high-throughput analysis and pave the way for further investigation using more complex therapeutic oligonucleotides.
Interestingly, there are two possible reasons for using ultra-short columns for ONs: (i) achieving ultra-fast separation (1 min or less), while maintaining a reasonable efficiency, provided that the flow rate is sufficiently high and (ii) increasing the overall resolution achievable in gradient mode (corresponding to Pmin) within a reasonable analysis time (few minutes). The second aspect has not yet been explored but will be investigated in an upcoming work.
- High-Throughput Chromatographic Separation of Oligonucleotides: A Proof of Concept Using Ultra-Short Columns Honorine Lardeux, Szabolcs Fekete, Matthew Lauber, Valentina D’Atri, and Davy Guillarme. Analytical Chemistry 2023 95 (27), 10448-10456. DOI: 10.1021/acs.analchem.3c01934