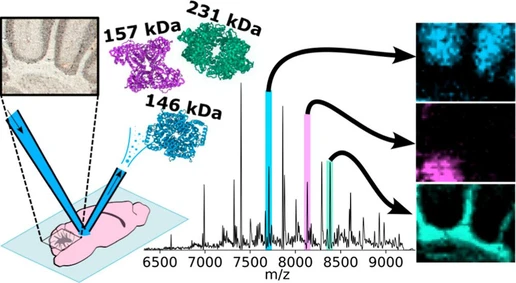
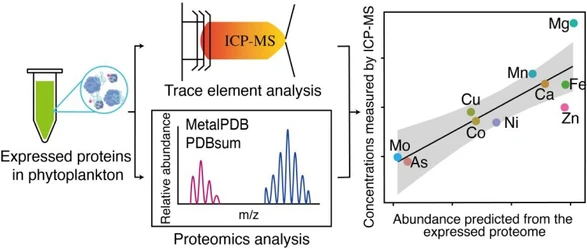
NPLC method to characterize end groups of poly lactic acid co-glycolic acid copolymers

- Photo: CAST: NPLC method to characterize end groups of poly lactic acid co-glycolic acid copolymers: Figure: (left) schematic illustration of the working principle of the NPLC separation. (right): key results obtained in the study
The CAST scientist Masashi Serizawa recently published a manuscript in which he investigated a novel method of using gradient elution normal-phase liquid chromatography with basic and acidic additives to separate PLGAs in the different end groups and in the different chemical compositions at the same time.
PLGA is an important material in drug delivery systems. It is used in nanoparticle-containing drugs to prevent a sudden increase in drug concentration in the body when the drug is ingested. The LA/GA ratio and differences in the terminal structure of PLGA have a significant effect on the degradation rate of PLGA in the body.
To distinguish these distinctions, we created a unique ternary gradient liquid chromatography method utilizing base and acid additives. Initially, we used a gradient of hexane, a poor solvent, and ethyl acetate, a good solvent, with a mobile phase containing a base additive to separate non-ester-terminated PLGAs (ester-terminated PLGA and cyclic PLGA) based on their chemical composition. Subsequently, by switching the mobile phase to THF containing an acid additive, we were able to elute acid-terminated PLGA.
This method offers the advantage of quick analysis compared to traditional NMR methods, making it potentially valuable for future industrial research. Furthermore, it can be applied to high molecular weight PLGA of 180 kDa, making it useful for the development of high molecular weight PLGA, which is challenging to analyze using mass spectrometry techniques such as MALDI-TOF-MS.
 CAST: Figure: (left) schematic illustration of the working principle of the NPLC separation. (right): key results obtained in the study.
CAST: Figure: (left) schematic illustration of the working principle of the NPLC separation. (right): key results obtained in the study.
The study is supported by the COAST/ TKI-Chemistry POLY-SEQU-ENCHY project between the UvA and Corbion (Gorinchem, The Netherlands) and is funded by Mitsubishi Chemical Corporation.