Functionality-type and chemical-composition separation of poly(lactide-co-glycolide) using gradient elution normal-phase liquid chromatography with basic and acidic additives

- Photo: Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 5. (a) NPLC separations in the difference of chemical composition. (b) NPLC separations in the difference of molecular weight. All analytical conditions were the same as in Fig. 4.
In the research article published recently in the Journal of Chromatography A, the researchers from the University of Amsterdam and from the Centre for Analytical Sciences Amsterdam, Amsterdam, the Netherlands, from Corbion, Gorinchem, the Netherlands and from Mitsubishi Chemical Corporation, Kanagawa, Japan introduced a novel normal-phase liquid chromatography method to analyze end-group functionality and chemical composition distribution in poly(lactide-co-glycolide) (PLGA) polymers, essential for drug delivery applications.
Unlike traditional methods limited by sample throughput or molecular weight, this technique efficiently separates PLGA up to 183 kDa while distinguishing mono-ester and di-acid terminated polymers and varying lactic acid/glycolic acid ratios. By utilizing a cross-linked diol column with a tailored ternary gradient, this approach provides detailed insights into PLGA’s end-group distribution and composition. This method offers valuable potential for advancing PLGA product development and quality control.
The original article
Functionality-type and chemical-composition separation of poly(lactide-co-glycolide) using gradient elution normal-phase liquid chromatography with basic and acidic additives
Masashi Serizawa, Jeroen Reekers, Pieter van Delft, Michel van Bruijnsvoort, Peter J. Schoenmakers, Andrea F.G. Gargano
Journal of Chromatography A, Volume 1730, 2024, 465137
https://doi.org/10.1016/j.chroma.2024.465137.
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Highlights
- A gradient NPLC method using basic and acid additives separates cyclic and acid-terminated PLGAs.
- The method was optimized to reduce the molecular weight dependency.
- Ester-terminated up to Mw 183.0 kDa and acid-terminated PLGA up to Mw 56.3 kDa were analyzed.
- Different lactic acid/Glycolic acid ratios of polymers of the same Mw were separated.
- End groups separation was confirmed by fractionation and SEC-HRMS.
Abstract
End groups of poly(Lactide-co-glycolide) (PLGA) play an important role in determining the properties of polymers for use in drug delivery systems. For instance, it has been reported that the encapsulation efficiency in PLGA microspheres varies significantly between ester-terminated and acid-terminated PLGA. More importantly, the in-vivo degradation time of such polymer excipients is influenced by the functional end-group of the copolymer used.
The end group distribution in PLGA polymers has been studied using electrospray and matrix-assisted laser-desorption/ionization – high-resolution mass spectrometry. In both cases, the application of these methods is typically limited to PLGA having a molecular weight of up to 4 kDa. 13Carbon-nuclear-magnetic-resonance has also been reported as a method to differentiate and quantify PLGA end groups with a molecular weight up to 136 kDa. However, reported NMR methods take over 12 h per sample, limiting throughput. Cryoprobe NMR can reduce the time required for the process, however such NMR equipment is costly, which makes it unsuitable for the quality control of PLGA.
Here, we present a normal-phase liquid chromatography method capable of resolving functionality type distribution (FTD) and, partially, chemical composition distribution (CCD) in commercial PLGA polymers obtained from ring opening polymerization. This method can separate PLGA polymers with a molecular weight of up to 183.0 kDa while also enabling the simultaneous separation of the difference of Lactic acid (LA)/Glycolic acid (GA) ratios.
To achieve this, a cross-linked diol column was used with a ternary gradient from HEX to 0.1 % v/v TEA in EA to 0.1 % v/v FA in THF to allow first for the elution of mono-ester terminated PLGA, followed by the di-acid terminated. In addition, a separation of ester-terminated PLGA in the difference of the LA/GA ratio was achieved.
This method is expected to aid in understanding the correlation between PLGA's FTD, CCD, and physical properties, facilitating product development and quality control.
1. Introduction
Poly(lactic-co-glycolic acid) (PLGA) is a biodegradable polyester composed of lactic acid and glycolic-acid monomers. PLGA is currently being studied for use in surgical sutures, absorbable implants, drug encapsulation, drug delivery, and tissue engineering because during degradation within the human body (hydrolyzing the ester bonds in the polymer chain), it breaks down into harmless and non-toxic compounds [[1], [2], [3], [4], [5]].
The distribution of functionality types and chemical compositions of PLGA plays a crucial role in controlling the various properties of the polymer [1,[6], [7], [8]]. It has been reported that there are differences in the amount of drugs that can be encapsulated and the ability to release drugs depending on the end groups of PLGA [8]. For instance, ester-terminated PLGA has been shown to have a significantly higher encapsulation efficiency than acid-terminated PLGA. Acid-terminated PLGA microspheres, on the other hand, release drugs faster than ester-terminated PLGAs. [8] Additionally, the LA/GA ratio of PLGA affects drug-release kinetics because of differences in water solubility and the glass-transition point (Tg) [9].
Understanding the functionality-type distribution and chemical-composition distribution of PLGA is crucial to clarify the correlation between the polymer's primary structure and the required physical properties. It is also essential from a quality perspective that PLGA excipients consistently meet the required performance. Titration is a cost-effective standard method to measure acid values of acid-terminated PLGA [10]. However, it cannot directly distinguish between acid-terminated PLGA and free acid (e.g. residual from the synthesis) and is insensitive to ester-terminated PLGA.
Various analytical methods such as matrix-assisted laser-desorption/ionization – high-resolution mass spectrometry (MALDI-HRMS), electrospray ionization – (tandem) mass spectrometry (ESI-MS or ESI-MS/MS), and nuclear-magnetic-resonance (NMR) spectroscopy are used for analyzing the chemical composition and terminal structure of PLGA [9,11]. However, all these methods have their limitations. MALDI-HRMS and ESI-MS(/MS) are suitable for distinguishing between cyclic and ester-terminated PLGA but are restricted to detecting molecular weights typically below, less than, or equal to 4 kDa. The ionization response may vary depending on the end group, complicating the process of quantification. Furthermore, these methods are ineffective in separating acid-terminated PLGA and ester-terminated PLGA when there are isomers with the same molar mass. (an example of isomers with the same molar mass is shown in Figure S1.)
NMR can differentiate between acid and ester-terminated PLGA by detecting the presence or absence of a terminal structure through 13C NMR analysis [9]. However, this method described by J. Sun et al. is time-consuming, requiring more than 12 h of analysis, and is typically not effective in detecting acid-terminated PLGA due to the low intensity of the carboxylic group as an end group in polymers. Additionally, even though a cryoprobe NMR can reduce the time required for the process, such NMR equipment is costly, which makes it unsuitable for the quality control of PLGA.
In conclusion, it is challenging to quantitatively analyze a mixture of acid-terminated PLGA and ester-terminated PLGA using the current methods. Therefore, it is of interest to develop methods that can offer an indication of the end group distribution in PLGA polymers, which could be used during, for instance, product development.
One approach to determine polymer end groups is using interactive polymer chromatography (IPC), in which retention in IPC is often dependent on the chemical composition, functionality, and molecular weight of the polymer [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21]]. More specifically, interaction gradient polymer elution chromatography (interactive GPEC) [17,[22], [23], [24]]. In interactive polymer LC, the retention is governed by the polymer interaction with the chemical groups of the chromatographic column's stationary phase, the polarity and elution strength of the mobile phase, and chemicophysical parameters such as the properties of the solvents used (e.g. theta solvent) and temperature. Normal phase and reversed phase gradient chromatography methods are often applied [[12], [13], [14], [15], [16], [17], [18], [19], [20]].
Previous research reported the use of interactive GPEC for the characterization of low molecular weight (<4000 Da) aromatic polyester from condensation reactions. Philipsen et al. used shallow gradients in interaction GPEC separations using normal-phase chromatography. The polyester samples were mainly speciated by their cyclic, OH-COOH, diol, and diacid structures, suppressing MW effects that were dominating in RPLC-based separations [25]. Further research from the same group systematically explored the impact of other LC method parameters on the method performance [24].
However, to date, there are no reports that simultaneously separate aliphatic polyesters, such as PLGA having different end groups and monomer ratios, and no reports describing interactive GPEC of polyesters with Mw > 5 kDa. Polymers with different end groups may be differentiated thanks to the differences in the interactions of the terminal structure (end groups) with the stationary phase. However, this becomes more challenging with polymers of higher molecular weight (e.g. above 5 kDa). This is because as the molecular weight increases, the ratio of the terminal structure to the total molecular weight decreases.
The present research aims to develop fast and efficient methods for separating components of PLGA based on their terminal structure and chemical composition. Ideally, such methods should be applicable across a broad range of compositions and molecular weights, extending the upper mass limit beyond what has previously been achieved. Finally, we explored the NPLC separation developed, collecting fractions and analyzing these via SEC-MS for the detailed characterization of the PLGA samples separation described.
2. Experimental
2.1. Chemicals and samples
For the NPLC and RPLC methods, the solvents used included n-hexane (HEX) (> 99.5 %, HiperSolv grade) obtained from VWR International(Leuven, Belgium). Ethyl acetate (EA) (>99.5 %, HPLC grade) was obtained from Acros Organics (Janssen-Pharmaceuticalaan, Belgium). Tetrahydrofuran (THF) (unstabilized, GPC grade) was obtained from Biosolve (Valkenswaard, The Netherlands). Methanol (MeOH) was obtained from Biosolve (Valkenswaard, The Netherlands). Triethylamine (TEA) (> 99.5 %, puriss p.p. grade) was obtained from Sigma-Aldrich (Darmstadt, Germany). Formic acid (FA) (> 98 %, puriss p.p. grade) was obtained from Sigma-Aldrich (Darmstadt, Germany).
For SEC, the solvents used included THF (unstabilized, GPC grade) obtained from Biosolve (Valkenswaard, The Netherlands). FA (> 98 %, puriss p.p. grade) was obtained from Sigma-Aldrich (Darmstadt, Germany).
For mass spectrometry, the solvents used included acetonitrile and water, both obtained from Biosolve (Valkenswaard, The Netherlands). Cesium iodide (CsI) was used as an ionization agent (>99.5%) obtained from Sigma-Aldrich (Darmstadt, Germany). To calibrate the SEC separation, polystyrene standards were obtained from Polymer Laboratories Inc (California, USA).
The samples used in this study are commercially available as PURASORB® and were kindly provided by Corbion (Gorinchem, The Netherlands). The polymers are obtained via ring-opening polymerization, the polymerization reaction in which glycolide and lactide react to create a statistical copolymer consisting of a block lactic acid and glycolic acid [[26], [27], [28]]. The properties of the polymers used in this study are summarized in Table 1
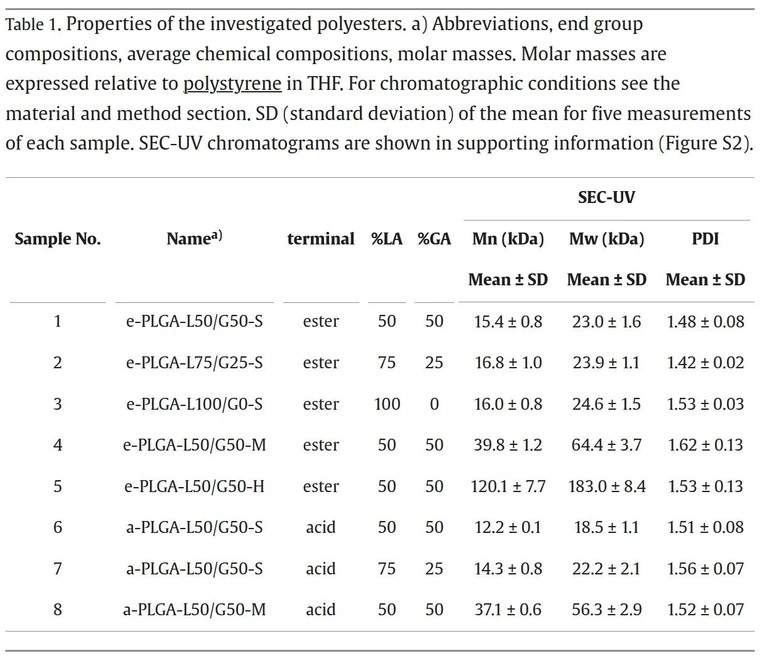 Journal of Chromatography A, Volume 1730, 2024, 465137: Table 1. Properties of the investigated polyesters. a) Abbreviations, end group compositions, average chemical compositions, molar masses. Molar masses are expressed relative to polystyrene in THF. For chromatographic conditions see the material and method section. SD (standard deviation) of the mean for five measurements of each sample. SEC-UV chromatograms are shown in supporting information (Figure S2).
Journal of Chromatography A, Volume 1730, 2024, 465137: Table 1. Properties of the investigated polyesters. a) Abbreviations, end group compositions, average chemical compositions, molar masses. Molar masses are expressed relative to polystyrene in THF. For chromatographic conditions see the material and method section. SD (standard deviation) of the mean for five measurements of each sample. SEC-UV chromatograms are shown in supporting information (Figure S2).
Depending on the polymerization conditions, the PLGA polymer is obtained with different end structures: cyclic, ester, and acid terminated. Ester-terminated PLGA is obtained by using aliphatic alcohol, whereas for acid-terminated PLGA, a hydroxy acid is used as an initiator of the polymerization (Fig. 1). To test the broad applicability of the developed NPLC method, samples were selected to span a wide range of molecular weights (with Mn between 13.5 kDa – 128.6 kDa), functionalities (ester-terminal and acid-terminal), and chemical compositions (LA/GA=50/50, 100/0).
 Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 1. Schematic representation of the structures of the studied poly(lactide-co-glycolide). The chemical structure of R shown in Figure 1 depends on the aliphatic alcohol used during polymerization.
Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 1. Schematic representation of the structures of the studied poly(lactide-co-glycolide). The chemical structure of R shown in Figure 1 depends on the aliphatic alcohol used during polymerization.
For NPLC method development, samples were prepared at a concentration of 6 mg/mL in EA. For SEC and SEC-MS method development, samples were prepared at a concentration of 1 mg/mL in THF.
2.2. Instrumentation
RPLC, NPLC, and NPLC fractionation experiments were performed using a Waters Acquity UPLC H Class system (Waters, Milford, MA, USA). The system comprised a quaternary solvent manager, sample manager, column manager, and ELS detector.
For SEC-MS/UV experiments, a similar Waters Acquity system was connected to a Waters Synapt G2 high-resolution mass spectrometer.
SEC-MS/UV experiment was carried out using the binary pump and PDA detector equipped with an analytical flow cell (Vdet = 500 nL).
2.3. Analytical conditions
2.3.1. RPLC and NPLC coupled to ELSD detection
RPLC and NPLC method development was carried out using the quaternary pump. A Thermo Scientific HIPERSIL C18 (150 × 2.1 mm i.d., 3.0 µm particles) (‘C18 column’) was used for RPLC analysis. A Phenomenex Luna HILIC (150 × 2.0 mm i.d., 3.0 µm particles) column (‘Diol column’) was used as NPLC column.
For the RPLC and the NPLC experiment, 1.0 µl of the solute was injected into the column operating at a flow rate of 0.2 mL/min and thermostatted at 23 °C. ELSD was performed using a 10-Hz acquisition rate and a set gain factor of 150, 40 psi N 2 gas pressure, a nebulizer set to cooling, and the drift tube operated at 70 °C [29].
In our initial gradient experiments, binary gradients using RPLC or NPLC methods were used.
A RPLC gradient method from MeOH (mobile phase A) to THF (mobile phase B). (tg/t0 of 7.2) following the gradient programming described in Table 2 was used.
 Journal of Chromatography A, Volume 1730, 2024, 465137: Table 2. LC method program for the NPLC and RPLC binary gradient method 1.
Journal of Chromatography A, Volume 1730, 2024, 465137: Table 2. LC method program for the NPLC and RPLC binary gradient method 1.
Samples were prepared at a concentration of 10.0 mg/mL in THF. 1.0 µl of the solute was injected into the column operating at a flow rate of 0.2 mL/min and thermostatted at 23 °C.
For the NPLC binary gradient, a gradient from HEX (mobile phase A) to THF (mobile phase B) was programmed following what described in table 2. The gradient time over dead time (tg/t0) of the gradient was 8.2.)Samples were prepared at a concentration of 10.0 mg/mL in THF. 1.0 µl of the solute was injected into the column operating at a flow rate of 0.2 mL/min and thermostatted at 23 °C.
A binary NPLC gradient method using EA as a mobile phase B and was also tested following the same conditions as reported for the gradient HEX to THF., With these conditions, the samples were prepared at a concentration of 10.0 mg/mL in EA. 1.0 µl of the solute was injected into the column operating at a flow rate of 0.2 mL/min and thermostatted at 23 °C.
Results from the binary gradients in RPLC and NPLC are in Figure S3–5 of the supporting information.
In NPLC, three ternary gradients were tested.
The gradient programming of ternary gradient 1 is described in Table 3.
 Journal of Chromatography A, Volume 1730, 2024, 465137: Table 3. LC method program for the NPLC ternary gradient method 1.
Journal of Chromatography A, Volume 1730, 2024, 465137: Table 3. LC method program for the NPLC ternary gradient method 1.
Three different mobile phases were used: HEX (mobile phase A), EA (mobile phase B), and THF (mobile phase C). (tg/t0 first 7.2, second gradient 0.5.)
The gradient programming ofternary gradients 2 and 3 are described in Table 4. Mobile phases: HEX (mobile phase A), EA with 0.1 % v/v TEA (mobile phase B), and THF with 0.1 % v/v FA (mobile phase C) were used. In the ternary gradient 2, the tg/t0 in the first gradient was 1.7, and the tg/g0 in the second gradient was 0.5. In the ternary gradient 3, tg/t0 in the first gradient was 0.2, and the tg/g0 in the second gradient was 0.5.
 Journal of Chromatography A, Volume 1730, 2024, 465137: Table 4. LC method program for the NPLC ternary gradient methods 2 and 3.
Journal of Chromatography A, Volume 1730, 2024, 465137: Table 4. LC method program for the NPLC ternary gradient methods 2 and 3.
For the ternary gradient analysis, samples were prepared at a concentration of 1.0 mg/mL in EA. 6.0 µl were injected into the column operating at a flow rate of 0.2 mL/min and thermostatted at 23 °C. For termary gradient 3 the column was alsothermostatted at 55 °C.
2.3.2. NPLC fractionation
For the NPLC fractionation experiment, samples were prepared at a concentration of 1.0 mg/mL in EA. 10.0 µl of the solute was injected into the column operating at a flow rate of 0.2 mL/min and thermostatted at 55 °C. Ternary gradient method 3 was employed using HEX (mobile phase A), EA with 0.1 % v/v TEA (mobile phase B), and THF with 0.1 % v/v FA (mobile phase C). Fractions were collected between 4.5 and 6.0 min five times from a line to ELSD operating the ternary gradient method 3.
2.3.3. SEC-UV characterization of PLGA copolymers and SEC‑MS/UV characterization of NPLC fractions
SEC-UV characterization of the PLGA polymers was carried out using an Agilent PLgel MiniMIX-D (250 × 4.6 mm i.d., 5.0 µm particles). Unstabilized THF with 0.1 % FA was used as the mobile phase. The separation was carried out at 0.2 mL/ min. Samples were dissolved in THF, and 5.0 µL were injected. Column calibration and estimation of Mn and Mw were done using polystyrene standards (Mw 1880.0 kDa, 679.0 kDa, 382.0 kDa, 114.2 kDa, 29.3 kDa, 10.0 kDa, 7.0 kDa, 3.3 kDa, 1.7 kDa, and 0.6 kDa) and toluene.
The SEC-MS/UV analysis was carried out using an Agilent PLgel MesoPore (250 × 2.1 mm i.d., 3.0 µm particles) column.
5.0 µL of solute was injected onto the SEC column set operated at an F of 0.04 mL/min THF containing 0.1 % (v/v) FA and thermostatted at a temperature of 23 °C. Using restriction capillaries, the effluent was split 1:1 to the UV and HRMS detectors, respectively. At the diverter valve of the mass spectrometer, a make-up flow containing the ionization agent(s) was infused to create a total flow to the ESI inlet of 20 µL/min, with a 1:1 ratio of analytical and make-up flow. HRMS conditions were: m/z range, 600–5000; scan time, 1 s; positive ESI; time-of-flight MS Resolution mode; capillary voltage, 3.5 kV; sampling cone, 200 V; trap collision energy, 10 eV; source temperature 120 °C; desolvation temperature, 350 °C; desolvation gas flow, 850 l/h; nebulizer gas flow, 100 l/h. Internal mass calibration was performed using CsI as reference mass [30]. Detection was performed at 220 nm with a bandwidth of 4.8 nmand a scan rate of 20 Hz.
For parallel UV/HRMS detection, the analytical effluent was split after the SEC column-set using a tee-piece and in-house made restriction capillaries (450 × 0.025 mm i.d.), ensuring a split ratio of 1:1 to the diode-array and mass spectrometer, respectively. Using the diverter valve of the Synapt-G2 system, the analytical flow from the SEC separation was combined with a make-up B flow Consisting of 10 % v/v 1 mM CsI aq in acetonitrile.
Instrument control, data acquisition, and data processing of all experiments were achieved with the MassLynx 4.1 program (Waters).
2.4. Data and data analysis
RPLC and NPLC data were acquired using Empower software (Waters, version 3). Peak lists derived from chromatograms were extracted from Empower software.
The SEC-MS/UV data was acquired using MassLynx software (Waters, version 4.1). Peak lists derived from chromatograms and mass spectra were extracted from MassLynx 4.1.
Origin 2019b software was used to visualize normal phase liquid chromatograms, size exclusion chromatograms, and mass spectra. For visualization of all chromatograms collected in this study, the raw data were smoothed using a Savitzky-Golay finite impulse response (FIR) smoothing filter of 3rd polynomial order and a window of 50 points.
To obtain the number average (Mn), mass average molar mass (Mw), polydispersity index (PDI), and extracted SEC curves from individual distributions present in the SEC-UV data, regions of interest were defined, extracted, and summed on the 2D axis. The time axis was then transformed to molecular weight according to the SEC calibration, and the raw data was used to determine Mn, Mw, and PDI.
The Mn and Mw values were calculated using the following equations where S is the signal and M the mass:
\(\bar{M_n} = \frac{\sum (S_t*M_t)}{\sum S_t}\)
\(\bar{M_w} = \frac{\sum (S_t*M_t)*M_t}{\sum (S_t*M_t)}\)
\(PDI = \frac{M_w}{M_n}\)
For SEC-MS data analysis SEC data between 12.0 min and 13.0 min were averaged. Data were exported as .csv. MSPolyCalc was used for the identification of detected peaks in mass spectra [31].
3. Results and discussion
In our investigation, we aimed to explore the use of interaction gradient polymer elution chromatography (GPEC) to separate cyclic, acid, and ester-terminated PLGA copolymers obtained by ring-opening polymerization. The structures of the polymers, as well as molecular weight and other characteristics, are reported in Fig. 1 and Table 1. In our discussion, the aliphatic ester and free carboxylic acid end group, as shown in Fig. 1, will from hereon be referred to as ester- and acid end groups, which will be indicated as ester and acid-terminated, respectively.
3.1. Developing an NPLC method for PLGA end group separations
3.1.1. Comparison between interactive GPEC using RPLC and NPLC
In our study, we first compared an interactive GPEC using RPLC and one using NPLC. Figure S3 displays the comparison of NPLC chromatograms between ester-terminated and acid-terminated PLGA with identical molecular weights (Mw of 23.0 kDa and 18.5 kDa, sample 1 and 6 of Table 1, respectively). We noticed that some PLGAs were eluted around 2.0 min(T0) in this method. Between 5.0 and 20.0 min, we observed that both acid and ester-terminated PLGA samples had broad distributions when subjected to RPLC. This outcome is thought to be influenced by the molecular weight of the polymers. When acid-terminated and ester-terminated PLGA with the same molecular weights were compared, retention times showed little difference. The broad distribution seen in RPLC is believed that molar mass effects can mainly dominate the separation. These results indicate that interactive GPEC using RPLC is not effective in separating ester and acidic end groups of PLGA [25].
Therefore, we tested interactive GPEC based on NPLC. HEX was chosen as a poor solvent present at a high% in the initial part of the analysis. This is because HEX can precipitate PLGA with a wide range of molecular weights.In our experiments, 40% HEX in EA induced precipitatation of solutions of polymers in EA. To determine a good solvent, we tested THF and EA based on their solvent strength and solubility (Figure S4) [32]. Dichloromethane was not considered a good solvent for this test due to the poor solubility of PLGA, particularly for PLGA with high molecular weight. For all methods, the same gradient changes (100 % in 15 min) were used (tg/t0 of 7.2), and the solvent used to solubilize the polymers was EA.
When comparing NPLC using THF as a mobile phase B to that using EA as a mobile phase, EA as a mobile phase B was found to be more effective in separating end group differences in 18.5 kDa and 23.0 kDa PLGAs (results presented in Figure S4). However, when this method was applied to PLGAs with 52 kDa and 64.4 kDa, ester-terminated PLGA and acid-terminated PLGA were found to overlap. In addition, Figure S4 (c) showed that acid-terminated PLGA with 18.5 kDa had lower intensity than ester-terminated PLGA with 23.0 kDa. To clarify if parts of acid-terminated PLGA were not eluted, we tested ternary gradient methods using HEX/EA/THF as THF has higher elution strength in NPLC than EA. (Fig. 2) A similar ternary gradient method was previously used as a modifier in Philipsen et al.'s method for aromatic polyesters [25].
 Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 2. NPLC separations using the ternary gradient 1. (a) for ester-terminated PLGA and acid-terminated PLGA with 23.0 kDa and 18.5 kDa, respectively. (b) for ester-terminated PLGA and acid-terminated PLGA with 64.4 kDa and 52 kDa, respectively. Column: Diol column, eluent: HEX (mobile phase A) to EA (mobile phase B)), and THF (mobile phase C). The ternary gradient 1 was programmed using the gradient method described in Table 2. Temperature:23 °C, flow: 0.2 mL/min, injection: 1.0 µl, concentration: 10 mg/ml in EA, detection: ELSD.
Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 2. NPLC separations using the ternary gradient 1. (a) for ester-terminated PLGA and acid-terminated PLGA with 23.0 kDa and 18.5 kDa, respectively. (b) for ester-terminated PLGA and acid-terminated PLGA with 64.4 kDa and 52 kDa, respectively. Column: Diol column, eluent: HEX (mobile phase A) to EA (mobile phase B)), and THF (mobile phase C). The ternary gradient 1 was programmed using the gradient method described in Table 2. Temperature:23 °C, flow: 0.2 mL/min, injection: 1.0 µl, concentration: 10 mg/ml in EA, detection: ELSD.
In Fig. 2, it was observed that a portion of acid-terminated PLGA with a molecular weight of 18.5 kDa eluted in a small amount the area between 10 and 15 min and gave a major peak at approximately 25.0 min, which coincided with the mobile phase transition from EA to THF. On the other hand, acid-terminated PLGA with a molecular weight of 52 kDa did not exhibit a prominent peak around 25.0 min. These results suggested that acid-terminated PLGA with lower molecular weight had a stronger interaction with the stationary phase. To summarize, the ternary NPLC method (i) was able to separate ester-terminated and acid-terminated PLGA with a molecular weight of 18.5 kDa and 23.0 kDa but polymers of 52 kDa and 64.4 kDa presented co-elution of the two end groups. (ii) two elution areas were observed for acid-terminated PLGA with a molecular weight of 18.5 kDa.
The findings suggest that simply optimizing the choice of solvent is insufficient for distinguishing between PLGAs with various end groups covering a broad range of molecular weights. This is because the impact of the polarity of the end groups on the PLGAs being examined becomes less significant as their molecular weight increases.
3.1.2. Using additives to modulate the dissociation of acid-terminated PLGA in NPLC
In the previous chapter, we found that the effect of molecular weight is greater than the effect of the polarity of the end group of PLGA. In this chapter, we first investigated the effect of a steep gradient method to reduce molecular weight dependency in ternary NPLC analysis. Afterward, to increase the polarity difference between end groups, we investigated the impact of adding acid or base to the mobile phase using a steep gradient.
In Figure S5, the ternary gradient method 2 significantly reduced the molecular weight dependency for ester-terminated PLGAs compared to the ternary gradient method 1, shown in Fig. 2. However, under these conditions, only a small portion of the acid-terminated PLGA eluted. To understand the reason for the low intensity of the acid-terminated PLGAs, we investigate the influence of adding acidic additives to the mobile phase to reduce the strong interaction between the acid end group and the polar stationary phase.
In Fig. 3(a), the results were obtained by adding 0.1 % v/v of FA to both mobile B (EA) and mobile phase C (THF). On the other hand, Figure 3(b) shows the results obtained by adding 0.1 % v/v of TEA to mobile B (EA) and 0.1 % v/v of FA to mobile phase C (THF).
 Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 3. (a) NPLC separations using ternary gradient 2, adding FA to the mobile phase C. Column: Diol column, eluent: HEX (mobile phase A) to 0.1% v/v FA in EA (mobile phase B)), and 0.1% v/v FA in THF (mobile phase C). Temperature:23 °C, flow: 0.2 mL/min, injection: 6.0 µl, concentration: 1.0 mg/ml in EA, detection: ELSD. Figure 3(b). NPLC separations using ternary gradient 2, adding TEA to the mobile phase B and FA to the mobile phase C. The ternary gradient 2 was tested using HEX (mobile phase A), 0.1% v/v TEA in EA (mobile phase B), and 0.1% v/v FA in THF (mobile phase C). Other analytical conditions were the same as in Figure 3(a).
Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 3. (a) NPLC separations using ternary gradient 2, adding FA to the mobile phase C. Column: Diol column, eluent: HEX (mobile phase A) to 0.1% v/v FA in EA (mobile phase B)), and 0.1% v/v FA in THF (mobile phase C). Temperature:23 °C, flow: 0.2 mL/min, injection: 6.0 µl, concentration: 1.0 mg/ml in EA, detection: ELSD. Figure 3(b). NPLC separations using ternary gradient 2, adding TEA to the mobile phase B and FA to the mobile phase C. The ternary gradient 2 was tested using HEX (mobile phase A), 0.1% v/v TEA in EA (mobile phase B), and 0.1% v/v FA in THF (mobile phase C). Other analytical conditions were the same as in Figure 3(a).
The addition of FA to mobile phase B and C helped us to detect clear peaks of acid-terminated PLGA.
Interestingly, we observed that acid-terminated PLGAs were eluted at almost the same time as ester-terminated PLGAs, around 7.5 min in the elution area of 100 % EA. No polymer appears to elute with 100 % THF. We believe that the acid group of FA has a strong interaction, which is hydrogen-bridging, with the polar stationary phase when adding FA to the mobile phase B and C, leading to similar elution as the ester-terminated PLGA reducing the polar interaction with the stationary phase via the acid end group. Although the ternary gradient with adding FA could not separate between ester-terminated and acid-terminated PLGA, these results indicated that controlling the protonation of acid groups in polymers plays an important role in changing the retention behavior of acid-terminated PLGA. Therefore we tested if the addition of a base would increase the de-protonation of the acidic PLGA and increase its retention [33]. This was tested using a ternary gradient method adding a base (TEA) to EA and adding an acid (FA) to THF (Figure 3(b)).
The study conducted revealed that there was a clear distinction between ester-terminated and acid-terminated PLGAs. The use of TEA resulted in enhanced dissociation of the acid end groups. It was also noted that TEA helps shield diol OH and free silanols of the stationary phase, reducing repulsion effects. This phenomenon is expected to occur more prominently in non-protic organic solvents than in aqueous solvents. Due to deprotonation, acid-terminated PLGAs had a strong interaction with the stationary phase. However, upon changing the mobile phase from 0.1 % v/v TEA in EA to 0.1 % v/v FA in THF, the acid-terminated PLGA became protonated and could be detected between 12 and 15 min.
In this method, TEA played a key role in increasing the interaction between the stationary phase and the acid-terminated PLGA. When ternary gradient 3 was employed using the mobile phase as HEX, 0.1% v/v TEA in EA, and 0.1% v/v TEA in THF, acid-terminated PLGAs were not eluted. (Figure S6) Acid-terminated PLGAs were not eluted when TEA was added to THF. It seems that if the analysis is carried out using a base in the mobile phase, the acid-terminated PLGA will not elute using THF. Therefore, it is important to replace the TEA in EA with FA in THF - not only to achieve the desired polymer separation but also to maintain the stability of the diol column.
Lastly, to reduce the molecular weight dependency in the separation and obtain a sharper elution zone, steeper gradients were employed changing the mobile phase composition in only 0.5 min to 100 % EA, keeping this composition for 4.0 min and then changing it in 1 min to 100 % THF. In addition, we observed that PLGA with a molecular weight of 183.0 kDa has relatively low solubility in EA. Hence, while analyzing it with the thermostat set at 23 °C, some components that did not elute in EA were detected in the mobile phase C region (Supporting Information Figure S7). For a broader analysis of PLGA, we set the column thermostat at 55 °C to dissolve ester-terminated PLGA with higher molecular weights. These conditions were applied to PLGA polymers up to 183.0 kDa (Fig. 4). This elution strategy allowed to obtain polymer peak widths of less than 1 min, increasing peak heights significantly. This is expected to happen because introducing the heated mobile phase into the ELSD promotes the evaporation of the solvent, thereby improving the detection sensitivity.
 Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 4. NPLC separations to study the effects of the ternary gradient 3. Column temperature: 55 °C. Other analytical conditions were the same as in Figure 3(b).
Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 4. NPLC separations to study the effects of the ternary gradient 3. Column temperature: 55 °C. Other analytical conditions were the same as in Figure 3(b).
3.2. Application to the study of chemical composition distribution analysis of PLGA samples with different LA/ GA ratios
In the preceding sections, it was demonstrated that the NPLC technique can distinguish between PLGAs that have LA/GA ratios of 50/50 based on differences in their end groups. In order to investigate whether the retention in the NPLC method is affected by the chemical composition of PLGAs, PLGAs with comparable molecular weight distributions but varying chemical compositions were analyzed.
Fig. 5(a) shows chromatograms of ester-terminated PLGA with different chemical compositions analyzed under the ternary gradient 2 conditions. PLGA alternated co-polymers with LA/GA=100/0, 75/25, and 50/50 with a molecular weight of 24.6 kDa, 23.9 kDa, and 23.0 kDa eluted at 6.2 min, 6.6 min, and 7.1 min, respectively. This result shows that increasing the amount of GA in the co-polymer increases the retention and that it is possible to separate components of ester-terminated PLGA based on differences in chemical composition if their molecular weights are approximately the same. Interestingly, a similar influence in retention is observed when analyzing the PLGAs with LA/GA=50/50 with a molecular weight of 23.0 kDa, 64.4 kDa, and 183.0 kDa, as shown in Fig. 5(b). We speculate that this can be a result of molecular weight effects that result in differences in solubility in EA.
 Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 5. (a) NPLC separations in the difference of chemical composition. (b) NPLC separations in the difference of molecular weight. All analytical conditions were the same as in Fig. 4.
Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 5. (a) NPLC separations in the difference of chemical composition. (b) NPLC separations in the difference of molecular weight. All analytical conditions were the same as in Fig. 4.
To reduce the effect of molecular weight in the separation, a ternary gradient method (method 3) that includes a steep gradient in the first gradient (tg/ to of 1.7 whereas in method 2 this was 7.2) is illustrated in Figure S8. We examined whether this same method, as shown in Fig. 6, can be used to comprehend the chemical composition distribution of ester-terminated PLGA.
 Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 6. (a) NPLC separations in the difference of chemical composition using the ternary gradient 3, adding TEA to the mobile phase B and FA to the mobile phase C. The ternary gradient 3 was programmed as the gradient method described in Table 4. (b) NPLC separations in the difference of molecular weight using the same ternary gradient method as (a). Column: Diol column, Temperature:23 °C, flow: 0.2 mL/min, injection: 6.0 µl, concentration: 1 mg/ml in EA, detection: ELSD.
Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 6. (a) NPLC separations in the difference of chemical composition using the ternary gradient 3, adding TEA to the mobile phase B and FA to the mobile phase C. The ternary gradient 3 was programmed as the gradient method described in Table 4. (b) NPLC separations in the difference of molecular weight using the same ternary gradient method as (a). Column: Diol column, Temperature:23 °C, flow: 0.2 mL/min, injection: 6.0 µl, concentration: 1 mg/ml in EA, detection: ELSD.
Fig. 6 demonstrates that the ternary gradient method 3 can effectively separate ester-terminated PLGA based on the difference between the LA/GA ratio while minimizing the impact of molecular weight. The larger PLGA sample in our set presented still partial MW effects. We suggest that a solution similar to what Nielsen et al. reported [18] should be applied to further reduce this effect.
The chromatograms of acid-terminated PLGA with LA/GA ratios of 75/25 and 50/50 demonstrated almost identical peak shapes (Figure S8). Notably, the NPLC method is unsuitable for separating the differences in the chemical composition of acid-terminated PLGA.
3.3. SEC-MS analysis of fractions collected from the NPLC method
The NPLC method was developed using ester-terminated and acid-terminated PLGA. To better understand the separation process, we collected fractions from different parts of the chromatograms. We conducted an investigation to determine if the separation of polyesters by their different end groups, which Philipsen et al. did for aromatic polyesters, could also be applied to PLGAs using the NPLC method that we developed [24]. In their research, Philipsen et al. demonstrated that cyclic polyesters were eluted first, followed by diol, mono-acid, and di-acid terminated polyesters. With the help of SEC-MS analysis, we aimed to characterize the components of cyclic PLGA that were separated by the NPLC method using fractions that were collected during the NPLC method.
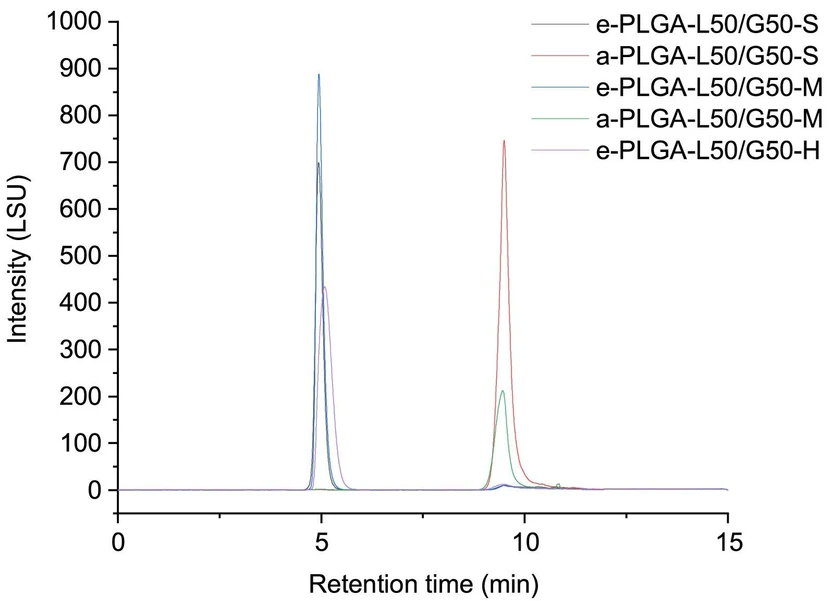
Fig. 7a shows a chromatogram of acid-terminated PLGA with a molecular weight of 18.5 kDa using a steep gradient. A peak was detected between 4.5 and 6.0 min; the MS spectrum obtained by fractionating this peak and analyzing it by SEC-MS is shown in Figure. 7(c). The size exclusion chromatograms are shown in Figure S9, and the mass spectra are shown in Figure S10. CsI was used as an additive for PLGA in SEC-MS to extend the ionization to a higher number of polymer repeating units. SEC-MS analysis indicated that the fractionated component was cyclic PLGA. The elution area overlapped with the ester-terminated peak.
 Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 7. (a) NPLC chromatogram of sample a-PLGA-L50/G50-S, with the fraction collected between 4.5 and 6.0 min indicated with dotted lines. Expanded SEC-MS mass spectra of the unfractionated (sample a-PLGA-L50/G50-S (b) and a fraction between 4.5 and 6.0 min (area corresponding to the elution of ester terminated PLGA) collected from sample a-PLGA-L50/G50-S (c), and a fraction between 8.5 and 10.0 min collected from sample a-PLGA-L50/G50-S (d). Expanded SEC-MS mass spectra (between m/z 1877 and 1905) of the unfractionated sample a-PLGA-L50/G50-S (e) and a fraction between 4.5 and 6.0 min (an area corresponding to the elution of ester-terminated PLGA) collected from sample a-PLGA-L50/G50-S (f), and a fraction between 8.5 and 10.0 min collected from sample a-PLGA-L50/G50-S (g). The assignment of each peak was supported by accurate mass measurements of product ions shown in Table S1. The assignment of each peak was supported by accurate mass measurements of product ions shown in Table S1.
Journal of Chromatography A, Volume 1730, 2024, 465137: Fig. 7. (a) NPLC chromatogram of sample a-PLGA-L50/G50-S, with the fraction collected between 4.5 and 6.0 min indicated with dotted lines. Expanded SEC-MS mass spectra of the unfractionated (sample a-PLGA-L50/G50-S (b) and a fraction between 4.5 and 6.0 min (area corresponding to the elution of ester terminated PLGA) collected from sample a-PLGA-L50/G50-S (c), and a fraction between 8.5 and 10.0 min collected from sample a-PLGA-L50/G50-S (d). Expanded SEC-MS mass spectra (between m/z 1877 and 1905) of the unfractionated sample a-PLGA-L50/G50-S (e) and a fraction between 4.5 and 6.0 min (an area corresponding to the elution of ester-terminated PLGA) collected from sample a-PLGA-L50/G50-S (f), and a fraction between 8.5 and 10.0 min collected from sample a-PLGA-L50/G50-S (g). The assignment of each peak was supported by accurate mass measurements of product ions shown in Table S1. The assignment of each peak was supported by accurate mass measurements of product ions shown in Table S1.
These results showed that this NPLC method can separate cyclic and acid-terminated PLGA. The NPLC method is expected to play an important role in identifying cyclic PLGA because they have properties different from those of the target linear acid-terminated PLGA.
4. Conclusion
Our study presents a new LC method that can effectively analyze differences in the terminal structure and chemical composition of PLGA. Our research has demonstrated the following capabilities of the new LC method: (i) The NPLC method, which employs an acid/base gradient, allows for the separation of acid-terminated PLGA from other PLGA in just 15 to 20 min. (ii) The method was tested with ester-terminated PLGA up to 183.0 kDa and acid-terminated PLGA up to 52 kDa, which is higher than methods based on mass spectrometry. (iii) The method can be used to separate ester-terminated PLGA with different chemical compositions. (iv) The method can separate and analyze cyclic PLGA contained in acid-terminated PLGA. The proposed analytical method was tested over a period of six months and delivered repeatable results.
We suggest that this analytical method can be used as a quality control measure during PLGA synthesis to monitor the correlation between PLGA's FTD, CCD, and track the PLGA hydrolysis reaction, and correlate these with the polymer physical properties. It is expected that this method will be applied in various applications beyond PLGA, such as tracking reactions when synthesizing composite polymers of acid-terminated PLGA along with other reactive polymers. It can also be used as a prototype for developing other polyester analysis methods and as a structural analysis method for acid-containing hydrophobic polymers other than polyester.
- Functionality-type and chemical-composition separation of poly(lactide-co-glycolide) using gradient elution normal-phase liquid chromatography with basic and acidic additives. Masashi Serizawa, Jeroen Reekers, Pieter van Delft, Michel van Bruijnsvoort, Peter J. Schoenmakers, Andrea F.G. Gargano. Journal of Chromatography A, Volume 1730, 2024, 465137. https://doi.org/10.1016/j.chroma.2024.465137.