How to improve the precision of analysis – semi-automated sample preparation for psychoactive compounds quantification in human plasma
- Photo: Bioanalytic: How to improve the precision of analysis – semi-automated sample preparation for psychoactive compounds quantification in human plasma.
Maciej Stopa 1,2, Julia Mironenka1, Rafał Szewczyk1,2, Anna Lenartowicz1, Adrian Soboń1,2, Katarzyna Krupczyńska-Stopa1,2, Andrzej Kwaśnica3
1. LabExperts sp. z o. o., Gdańsk, Poland; 2. Bioanalytic sp. z o. o., Gdańsk, Poland; 3. Lab4Tox sp. z o. o., Wrocław, Poland
Introduction
The precision, reproducibility and trueness of the result in forensic analysis is crucial. The accuracy of sample preparation during the psychoactive compounds quantitation is one of the key aspects. Automatic sample preparation minimize mistakes caused by poor performance of the analysis, discrepancies between analysts habits or different days of analysis. Accurate analytes detection relies on efficient extraction, low detection limits and maintaining the efficiency and cleanliness of LC-MS/MS system. Liquid-liquid extraction, by default, are less amenable for automation, and may be challenging because of large amount of sample preparation steps. This work shows the effective translation from manual method to semi-automated procedure of determination of 34 psychoactive compounds from human plasma using LC-MS/MS method.
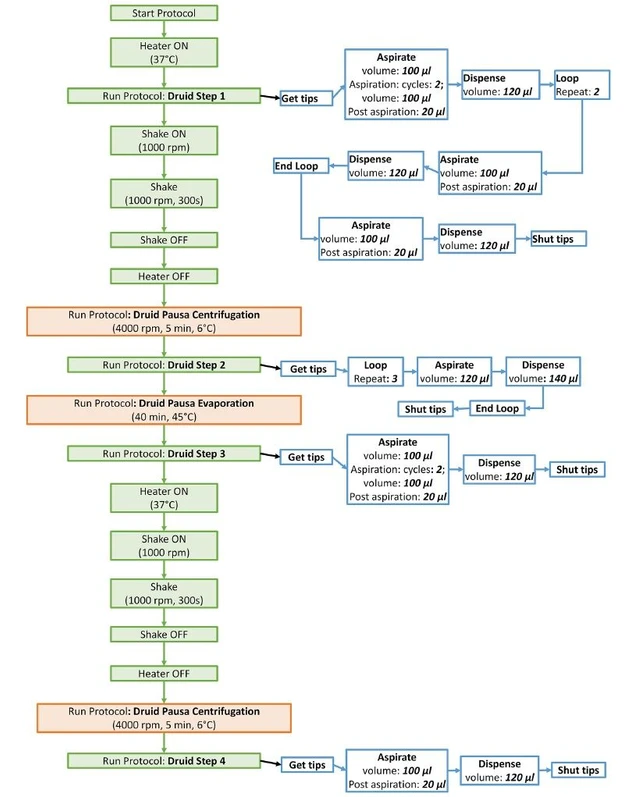 Bioanalytic: Figure 1 - Semi-automated sample preparation protocol using Hudson SOLO automatic pipette station with Hudson SOLO Soft and SoftLink V protocol Editor.
Bioanalytic: Figure 1 - Semi-automated sample preparation protocol using Hudson SOLO automatic pipette station with Hudson SOLO Soft and SoftLink V protocol Editor.
Methods
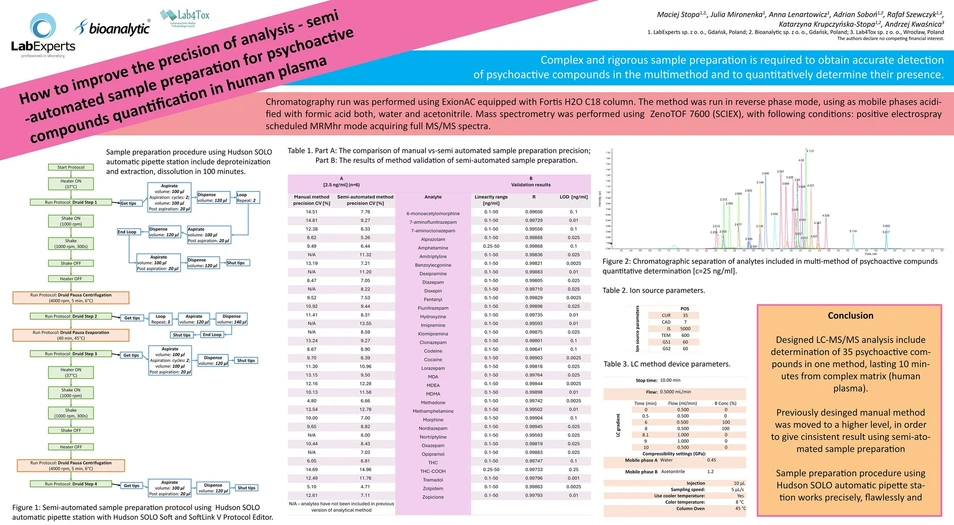
Liquid-liquid extraction is sufficient method to determine the amount of psychoactive compounds in human plasma. Sample preparation procedure developed using Hudson SOLO automatic pipette station with Hudson SOLO Soft and SoftLink V Protocol Editor include deproteinization and extraction, dissolution and transfer to a plate compatible with an LC autosampler in 100 minutes. Chromatography run was performed using ExionAC equipped with Fortis H2O C18 column. The method was run in reverse phase mode, using as mobile phases acidified with formic acid both, water and acetonitrile. Mass spectrometry was performed using ZenoTOF 7600 (SCIEX), with following conditions: positive electrospray scheduled MRMhr mode acquiring full MS/MS spectra. The data analysis including compound identification and quantification was performed with SciexOS 3.3 software.
 Bioanalytic: Table 1. Part A - The comparison of manual vs-semi automated sample preparation precision; Part B: The results of method of semi-automated sample preparation.
Bioanalytic: Table 1. Part A - The comparison of manual vs-semi automated sample preparation precision; Part B: The results of method of semi-automated sample preparation.
Preliminary data
Blood is easy to obtain information-rich matrix. It have only one disadvantage – it cannot be easily analyzed with LC-MS/MS. Preparation of a multimethod for psychoactive compounds determination which characterized by a different chemical properties requires complex and strict sample preparation to achieve accurate detection and compound presence confirmation. Unfortunately, in complex extraction steps are difficult to postpone due to various activities that has to be undertaken including – mixing, centrifugation, sample aspirations and evaporation. In this work, we show the possibility to transfer steps of aspiration, dilution, heating and mixing during the sample preparation on an automatic high precision pipette station. Other steps including centrifugation and evaporation were done manually. Beside the shortening of sample preparation time, which allows to perform the analysis of 96 samples in less than 2 hours, the results are subjected to less preparation error. Such configurations allow a substantial increase of sample throughput and convenience when compared to standard protocols. As a result of this translation, better repeatability (%CV ≤ 15%), maintaining a wide range of linearity (LLOQ-50 ng/ml) which met the validation criteria (R ≥ 0.995) of the method was achieved. An additional advantages of using automation – the analyst’s exposure to blood obtained from contractors is reduced. Also, automated protocol minimize the risk of losing valuable samples avoiding cases of human error.
![Bioanalytic: Figure 2: Chromatographic separation of analytes included in multi-method of psychoactive compounds quantitative determinantion [c=25 ng/ml].](https://lcms.labrulez.com/labrulez-bucket-strapi-h3hsga3/Bioanalytic_How_to_improve_the_precision_of_analysis_semi_automated_sample_preparation_for_psychoactive_compounds_quantification_in_human_plasma_Figure_2_Chromatographic_separation_1f0e513084_l.webp) Bioanalytic: Figure 2: Chromatographic separation of analytes included in multi-method of psychoactive compounds quantitative determinantion [c=25 ng/ml].
Bioanalytic: Figure 2: Chromatographic separation of analytes included in multi-method of psychoactive compounds quantitative determinantion [c=25 ng/ml].
Novel aspect
Automated method offers excellent repeatability and high accuracy of psychoactive compounds determination in human blood.