Development of temperature-controlled batch and 3-column counter-current protein A system for improved therapeutic purification
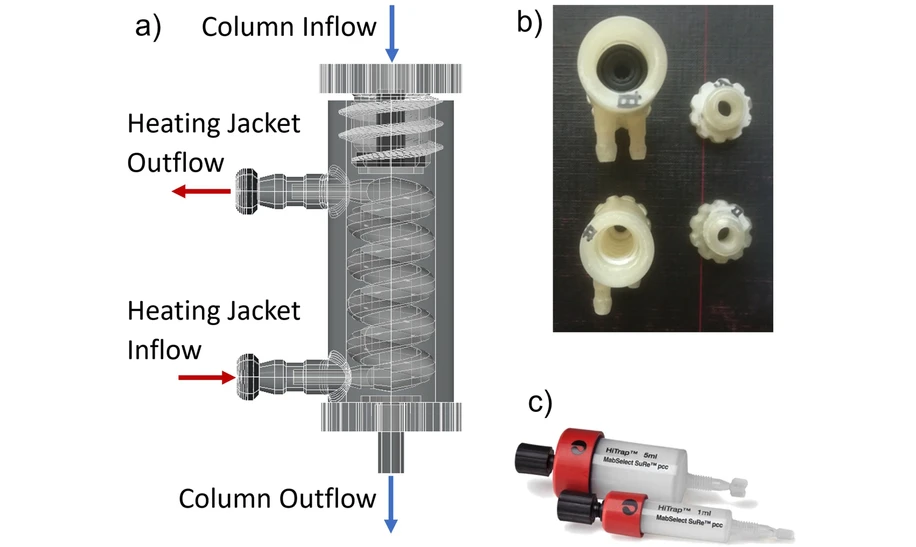
- Photo: Journal of Chromatography A, Volume 1730, 2024, 465110: Fig. 1. Overview of the heating jacket used to control the temperature of the MabSelect SuRe chromatography column. a) Shows the design of the heating jacket in AutoCAD. The heating jacket operates in counter-current mode. The water flowing through the heating jacket makes direct contact with the outside of the chromatography column. b) Shows the 3D printed heating jackets with and without a column inside (top: inside, bottom: empty). O-rings are used to prevent the heating jacket water from leaking out. c) The 1 mL columns used in the temperature-controlled chromatography experiments. The red cap was removed to allow the column to fit snugly within the heating jacket.
In the study published in the Journal of Chromatography A, researchers from the University College London, UK, and AstraZeneca, Cambridge, UK
investigated the impact of temperature on protein A chromatography for monoclonal antibody (mAb) purification, focusing on both batch and continuous counter-current systems.
Using a custom 3D-printed heating jacket, dynamic binding capacity (DBC) was evaluated at 10, 20, and 30 °C, revealing a significant DBC increase with temperature. Mechanistic and correlation-based models were developed to predict optimal operating conditions, enabling the design of a 3-column temperature-controlled periodic counter-current chromatography (TCPCC) system. Operating at 30 °C resulted in a 47% DBC increase and a two-fold productivity boost compared to 20 °C batch systems. Further productivity improvements were achieved by optimizing the TCPCC for varying feed concentrations, showing two-, three-, and four-fold increases for 1, 5, and 15 mg/mL mAb feeds, respectively. These findings highlight the utility of temperature-dependent models for optimizing chromatography systems to enhance efficiency and productivity.
The original article
Development of temperature-controlled batch and 3-column counter-current protein A system for improved therapeutic purification
Alexander Armstrong, Jorge Aranda Hernandez, Felix Roth, Daniel G. Bracewell, Suzanne S. Farid, Marco P․C․ Marques, Stephen Goldrick
Journal of Chromatography A, Volume 1730, 2024, 465110
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Highlights
- Process temperature is under-characterized for protein A chromatography.
- Novel temperature-controlled chromatography heating jacket designed and evaluated.
- Dynamic binding capacity increased with increasing temperatures.
- Productivity and resin utilization improved with increasing temperatures.
- The data were fit to correlation-based and mechanistic-based models.
Abstract
Maximizing product quality attributes by optimizing process parameters and performance attributes is a crucial aspect of bioprocess chromatography process design. Process parameters include but are not limited to bed height, eluate cut points, and elution pH. An under-characterized chromatography process parameter for protein A chromatography is process temperature. Here, we present a mechanistic understanding of the effects of temperature on the protein A purification of a monoclonal antibody (mAb) using a commercial chromatography resin for batch and continuous counter-current systems. A self-designed 3D-printed heating jacket controlled the 1 mL chromatography process temperature during the loading, wash, elution, and cleaning-in-place (CIP) steps. Batch loading experiments at 10, 20, and 30 °C demonstrated increased dynamic binding capacity (DBC) with temperature. The experimental data were fit to mechanistic and correlation-based models that predicted the optimal operating conditions over a range of temperatures. These model-based predictions optimized the development of a 3-column temperature-controlled periodic counter-current chromatography (TCPCC) and were validated experimentally. Operating a 3-column TCPCC at 30 °C led to a 47% increase in DBC relative to 20 °C batch chromatography. The DBC increase resulted in a two-fold increase in productivity relative to 20 °C batch. Increasing the number of columns to the TCPCC to optimize for increasing feed concentration resulted in further improvements to productivity. The feed-optimized TCPCC showed a respective two, three, and four-fold increase in productivity at feed concentrations of 1, 5, and 15 mg/mL mAb, respectively. The derived and experimentally validated temperature-dependent models offer a valuable tool for optimizing both batch and continuous chromatography systems under various operating conditions.
2. Materials, methods and theory
2.2. Methods
2.2.1. Tangential flow ultrafiltration concentration
The mAb was concentrated using tangential flow filtration (TFF). The TFF system was a Pellicon® XL 50 Device (EMD Millipore, Burlington, MA, USA) with an Ultracel® Ultrafiltration Membrane cassette (EMD Millipore, Burlington, MA, USA). The membrane cassette has a 10 kDa molecular weight cutoff (MWCO). 20 L of HCCF containing 0.18 mg/mL of mAb were concentrated to 1.3, 2.54, and 4.5 mg/mL for the respective studies. Samples of the permeate were collected for analysis. Following the mAb concentration, the solution was filtered through a 0.2 µm membrane to remove large impurities in the mAb sample.
2.2.2. Diafiltration buffer exchange of mAb sample prior to protein A chromatography
Buffer exchange was carried out on the concentrated mAb in the HCCF using a Pellicon® XL 50 Device (EMD Millipore, Burlington, MA, USA) with an Ultracel® Ultrafiltration Membrane (EMD Millipore, Burlington, MA, USA) cassette (10 kDa MWCO). Buffer exchange was carried out in a discontinuous mode. Initially, 250 mL of mAb in HCCF was added to the TFF feed vessel. This was followed by the additions of 250 mL, one diafiltration volume (DV), of equilibration to the feed vessel. The equilibration buffer used was the one buffer recommended by Cytiva (Marlborough, MA, USA) for MabSelect SuReTM resin (20 mM sodium phosphate, 0.15 M NaCl, pH 7.2). The solution was concentrated back down to 250 mL, and a 0.5 mL sample of the retentate and permeate were collected. The process was repeated for seven DVs to ensure 99.2% of the CHO media was removed. 0.5 mL samples of the retentate and permeate were collected following the addition of each DV. After the buffer exchange, the solution was filtered through a 0.22 µm membrane to remove any large impurities present in the buffer-exchanged mAb sample.
2.2.3. Protein G HPLC for determination of total mAb concentration
The high-performance liquid chromatography (HPLC) system used was an Agilent 1260 System (Agilent, CA, United States) with a degasser, solvent pump, injector, temperature controller and fixed wavelength (DAD) ultraviolet (UV) detector. A new 1 mL HiTrap® protein G column (Cytiva, Marlborough, MA, USA) was washed with 20% ethanol at 1 mL/min for 20 column volumes (CVs) to remove the storage preservatives. The column was then washed with 20 CVs of MilliQ water at 1 mL/min. Following the wash, the column was equilibrated with 20 CVs of binding buffer (20 mM sodium phosphate, pH 7.0). The assay was performed using a 2 mL/mL flow rate and a 100 µL injection volume. Glycine buffer (20 mM, pH 2.8) was used as the elution buffer. Samples were filtered with a 0.22 µm filter and centrifuged at 18,000 g for 10 min at 4 °C. The instrument method took 15 min per sample and was as follows: sample injection at time 0 min, binding buffer 0–5 min, elution buffer 5 to 10 min, binding buffer to re-equilibrate the column, 10–15 min. The mAb adsorption peak was detected using wavelength absorbance at 280 nm with a reference wavelength at 360 nm.
2.2.4. Size-exclusion (SE) HPLC for determination of mAb fragment, monomer, and aggregate concentration
The HPLC used was an Agilent 1260 System (Agilent, CA, United States), with a degasser, solvent pump, injector, temperature controller and fixed wavelength DAD UV detector. The column and guard column used were a Tosoh TSKgel UP-SW3000 4.6 × 300 mm 2.0µm size-exclusion column (Tosoh Biosciences, Tokyo, Japan) and TSKgel UP-SW3000 Guardcolumn (Tosoh). Samples were filtered with a 0.22 µm filter and centrifuged at 18,000 g for 10 min at 4 °C. The column was equilibrated for 10 CV at 0.2 mL/min with the mobile phase (100 mM sodium phosphate, 100 mM NaCl at pH 7.4). 20 µL of the sample was injected and separated under 0.2 ml/min of mobile phase for 30 minutes. The column was cleaned after every 10 samples with a solution of 100 mM sodium phosphate and 500 mM sodium chloride at pH 4. The column was re-equilibrated with the mobile phase for 10 column volumes. A protein standard mix of 15–670 kDa from Sigma-Aldrich (St. Louis, MO, USA) was used to create a standard curve to estimate the molecular weight.
2.2.5. Coupled protein A-size exclusion HPLC for single-step determination of mAb, fragments, aggregates, and host cell proteins (HCP)
In this study, we adapted a previous method by[23] Gjoka et al. (2014) which involved combining a POROS A20® Protein A column (Life Technologies, Carlsbad, Canada) with a TSKgel GW3000xL size exclusion column (Tosoh Biosciences, Tokyo, Japan) [23]. The columns were coupled together, with the protein A column in front of the size-exclusion column. A TSKgel GW3000xL Guardcolumn (Tosoh) was placed in front of both columns. The HPLC used was an Agilent 1260 System (Agilent, CA, United States) with a degasser, solvent pump, injector, temperature controller, and fixed wavelength DAD UV detector. The binding buffer used was 25 mM monobasic sodium phosphate, 300 mM NaCl, and pH 7.2. The elution buffer was 30 mM glycine, pH 2.8, with 300 mM NaCl. The assay was performed at a flow rate of 1 mL/min with an injection volume of 20 µL. The run time for the method was 45 minutes and was as follows: sample injection at time 0 min, binding buffer 0–15 minutes, elution buffer from 15–20 minutes, 20–45 minutes to complete the run, and re-equilibrate the column for the next run. A protein standard mix of 15–670 kDa from Sigma-Aldrich (St. Louis, MO, USA) was used to create a standard curve to estimate the molecular weight. The protein standard mix consists of 4 proteins: thyroglobulin bovine (0.5 g/L, MW ∼670,000 kDa), γ-globulins from bovine blood (1.0 g/L, MW ∼150,000 kDa), Albumin chicken egg grade VI (1.0 g/L, MW ∼44,300 kDa), Ribonuclease A type I-A from bovine pancreas (1.0 g/L, MW ∼ 13,700 kDa) and a low molecular weight marker p-aminobenzoic acid (0.01 g/L, MW 137.14 g/mol).
2.2.6. 96-well protein A chromatography resin experiments
PreDictor MabSelect SuRe Isotherm plates (Cytiva, Marlborough, MA, USA) containing 16-wells each of 50, 20, 8, 6, 4, and 2 µL of resin were incubated for 6 hours with 4.5 mg/mL of mAb. The flowthrough concentration was measured using protein G and protein A-SE HPLC. The amount of mAb bound to the resin at each concentration was determined, and a Langmuir isotherm was fit to the data. The Langmuir isotherm values were used in the models.
2.2.7. Design of a temperature-controlled batch chromatography system
A heating jacket for the 1 mL columns was initially designed using AutoCAD, as shown in Fig. 1a. This jacket is designed to fit the 1 mL column snugly, allowing water to flow around the column in a counter-current manner, thus facilitating direct contact with the column. The heating jacket was 3D printed using polylactic acid (PLA) plastic (Fig. 1b). The robustness of the heating jacket was tested, and it was found to be able to handle flow rates of greater than 2 L/min. The maximum flow rate of the 1 mL HiTrap MabSelect SuRe column was operated at was 1 mL/min (Fig. 1c). This setup enabled swift alterations to the column's temperature during operation. Initially, the columns were kept at a constant temperature (10, 20, or 30 °C) for all the chromatography steps: equilibration, loading, wash, elution, cleaning in place (CIP), and regeneration. The column residence time was 2.4 min. Once the optimal temperature was determined (30 °C for loading, 10 °C for all other steps), further runs were completed under these conditions.
 Journal of Chromatography A, Volume 1730, 2024, 465110: Fig. 1. Overview of the heating jacket used to control the temperature of the MabSelect SuRe chromatography column. a) Shows the design of the heating jacket in AutoCAD. The heating jacket operates in counter-current mode. The water flowing through the heating jacket makes direct contact with the outside of the chromatography column. b) Shows the 3D printed heating jackets with and without a column inside (top: inside, bottom: empty). O-rings are used to prevent the heating jacket water from leaking out. c) The 1 mL columns used in the temperature-controlled chromatography experiments. The red cap was removed to allow the column to fit snugly within the heating jacket.
Journal of Chromatography A, Volume 1730, 2024, 465110: Fig. 1. Overview of the heating jacket used to control the temperature of the MabSelect SuRe chromatography column. a) Shows the design of the heating jacket in AutoCAD. The heating jacket operates in counter-current mode. The water flowing through the heating jacket makes direct contact with the outside of the chromatography column. b) Shows the 3D printed heating jackets with and without a column inside (top: inside, bottom: empty). O-rings are used to prevent the heating jacket water from leaking out. c) The 1 mL columns used in the temperature-controlled chromatography experiments. The red cap was removed to allow the column to fit snugly within the heating jacket.
The flow rate used throughout the runs was 0.4 mL/min to achieve a residence time of 2.4 min. The fresh 1 mL (0.96 mL actual volume) HiTrap® MabSelect SuReTM column (Cytiva, Marlborough, MA, USA) was washed with 20 column volumes (CVs) of 20% ethanol to remove the preservatives from the storage solution. The column was then washed with 20 CVs of MilliQ H2O to remove the 20% ethanol. The column was placed in the temperature control jacket and connected to the water bath. The water bath was set to the desired temperature for the run (10, 20, and 30 °C). For each chromatographic run, the column was initially equilibrated with 5 CVs of equilibration buffer (20 mM sodium phosphate, 0.15 M NaCl, pH 7.2). The column was then loaded with 35 CVs of mAb feed at a concentration of 1.3 mg/mL to fully saturate the column with mAb. This was followed by a 4 CVs wash step using equilibration buffer (20 mM sodium phosphate, 0.15 M NaCl, pH 7.2). A step elution from pH 7.2 to pH 3.0 was carried out over 4 CVs followed by another 5 CV at pH 3.0 to ensure all the mAb was eluted from the column. CIP was performed by washing the column with 3 CVs of equilibration buffer to increase the column pH, followed by 6 CVs of 0.1 M NaOH for 15 min of contact time. The column was then re-equilibrated with 5 CVs of equilibration buffer. 1 mL fractions were collected throughout the duration of each chromatography run. UV spectras were collected every 0.5 s using a 8453 UV–visible Spectroscopy System (Agilent, Santa Clara, CA, USA).
2.2.8. Design of a temperature-controlled periodic counter-current chromatography (TCPCC) system
For the design of the TCPCC, one column was loaded with mAb feed at a concentration of 2.54 mg/mL at each temperature with a residence time of 1 min until full breakthrough occurred. This was followed by running two columns in series with a residence time of 1 min until breakthrough occurred. The experimental results were compared with the model predictions and used to determine the number of CVs of mAb to load during the TCPCC operation. Once the TCPCC operation parameters were determined, the three column TCPCC was designed and built (Fig. 2). Using four-way connectors and two-way valves, each column's heating jacket could have 10 or 30 °C water flow through it and back to the correct water bath. This allowed for the rapid cycling of the column temperature during the TCPCC runs (30 °C for loading, 10 °C for all other steps). For all steps except the loading step, the number of buffer CVs used was kept constant with the batch values.
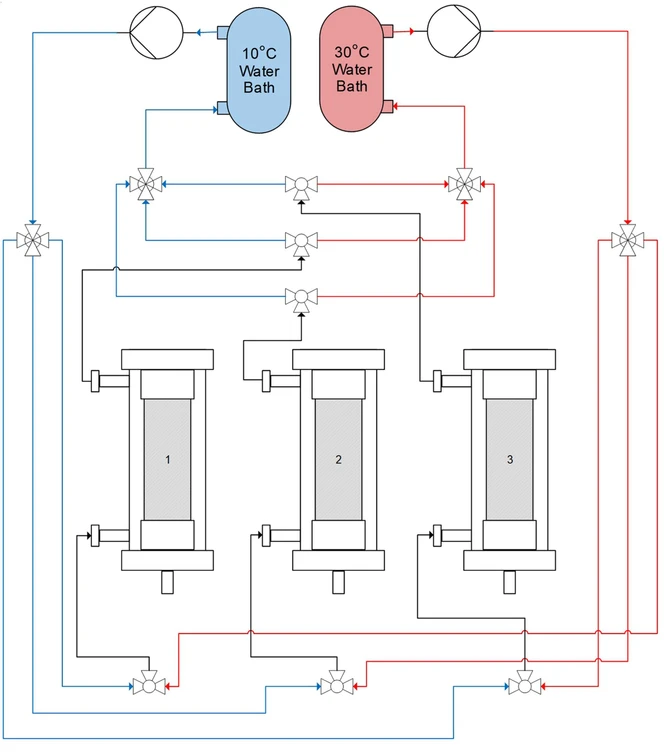 Journal of Chromatography A, Volume 1730, 2024, 465110: Fig. 2. Design of the temperature-controlled periodic counter-current chromatography (TCPCC) system. Two water baths, one at 10 and one at 30 °C, are connected to the three column heating jackets. The system of valves allows for either water temperature to flow through the heating jacket. The outlet valves on each column heating jacket allows for the water to be recirculated back to the correct water bath.
Journal of Chromatography A, Volume 1730, 2024, 465110: Fig. 2. Design of the temperature-controlled periodic counter-current chromatography (TCPCC) system. Two water baths, one at 10 and one at 30 °C, are connected to the three column heating jackets. The system of valves allows for either water temperature to flow through the heating jacket. The outlet valves on each column heating jacket allows for the water to be recirculated back to the correct water bath.
4. Conclusion
This paper demonstrates the effects of temperature on batch and continuous protein A chromatography using MabSelect SuRe. The DBC and OBC of batch and continuous chromatography systems were determined experimentally at 10, 20, and 30 °C. In all cases, increasing the temperature during loading led to higher DBC/OBC, productivity, resin utilization, and reduced buffer consumption. The experimental data were fit to correlation-based and mechanistic models. These models were used to predict, DBC/OBC, productivity, resin utilization, and buffer consumption over a range of residence times. The 3-column TCPCC system was run successfully for seven-cycles and matched the model predictions. The models were then used to predict productivity for batch and 3- column TCPCC systems at mAb feed concentrations of 1, 5, and 15 mg/mL. The productivity for the TCPCC system was further increased by increasing the number of columns for higher mAb feed concentrations. In the majority of cases, increasing the temperature led to better productivity. In all cases, the TCPCC had higher productivity than batch chromatography. Further work on the effect of temperature on protein A wash, elution, and CIP should be performed.
- Development of temperature-controlled batch and 3-column counter-current protein A system for improved therapeutic purification. Alexander Armstrong, Jorge Aranda Hernandez, Felix Roth, Daniel G. Bracewell, Suzanne S. Farid, Marco P․C․ Marques, Stephen Goldrick. Journal of Chromatography A, Volume 1730, 2024, 465110.