Oligos Made Easy - Part 2: AEX

KNAUER: Oligos Made Easy - Part 2: AEX
Charged for Action: The Hidden Secrets of AEX Oligonucleotide Analysis
Oligonucleotides—short, well-defined fragments of DNA or RNA—have become indispensable tools in modern life sciences. They underpin cutting-edge applications in molecular diagnostics, gene therapy, and pharmaceutical development. Before oligonucleotides can be confidently used in research or clinical settings, however, their quality must be rigorously assessed. Purity, sequence integrity, length distribution, and chemical modifications all play a decisive role in their performance.
 KNAUER: Oligos Made Easy - Part 2: AEX
KNAUER: Oligos Made Easy - Part 2: AEX
Among the analytical techniques available today, anion exchange chromatography (AEX) stands out as a robust and highly informative method for characterizing oligonucleotides. By exploiting their intrinsic negative charge, AEX provides detailed insight into product composition and impurity profiles that are difficult to obtain by other approaches.
How Scientists Read Oligonucleotides Using Charge
At the core of AEX lies a simple but powerful principle: oligonucleotides carry a negatively charged phosphate backbone. This charge increases in a predictable way with strand length and, to a lesser extent, sequence composition. Anion exchange chromatography uses this property to separate oligonucleotides based on differences in charge strength and interaction with a positively charged stationary phase.
As a result, AEX has become one of the most widely used analytical tools for evaluating oligonucleotide quality. It allows scientists to distinguish full-length products from truncated species, degradation products, or closely related variants—often with single-nucleotide resolution.
Why Ion Exchange Works: Binding, Retention, and Release
Ion exchange chromatography (IEX) relies on electrostatic interactions between analytes and a charged stationary phase. In the case of oligonucleotides, anion exchange is used, where negatively charged molecules interact with positively charged functional groups on the resin—most commonly quaternary amines.
The system consists of two main components:
- Stationary phase: A positively charged resin that binds anionic analytes.
- Mobile phase: An aqueous buffer system, typically combined with a salt gradient, that controls both pH and elution strength.
Retention in AEX is governed primarily by charge. Molecules with weaker overall charge interact less strongly with the stationary phase and elute earlier, while more highly charged oligonucleotides bind more tightly and require stronger elution conditions.
 KNAUER: Oligos Made Easy - Part 2 AEX: Figure 1 IEX overview including elution order dependency on analyte charge.
KNAUER: Oligos Made Easy - Part 2 AEX: Figure 1 IEX overview including elution order dependency on analyte charge.
The Five-Step Workflow of Ion Exchange Chromatography
AEX separations generally follow a well-established sequence of steps:
- Column equilibration
The column is conditioned with the starting buffer until the baseline and pH are stable. This ensures that all charged sites on the resin are ready for interaction. - Sample loading
Samples, prepared in the starting buffer, are introduced under conditions that promote binding of the target oligonucleotides while minimizing retention of unwanted impurities. - Washing
The column is washed with the starting buffer to remove uncharged or weakly interacting components. The baseline stabilizes as these species are flushed away. - Elution
Bound oligonucleotides are released using a salt or pH gradient. Increasing ionic strength introduces competing ions that displace the analytes from the resin. Weakly bound species elute first; strongly charged oligonucleotides elute later. - Regeneration and re-equilibration
A high-salt buffer removes any remaining bound material. The column is then returned to starting conditions in preparation for the next analysis.
 KNAUER: Oligos Made Easy - Part 2 AEX: Figure 2 Five steps of IEX procedure (in blue), outlined using an example chromatogram (in red).
KNAUER: Oligos Made Easy - Part 2 AEX: Figure 2 Five steps of IEX procedure (in blue), outlined using an example chromatogram (in red).
Why AEX Is Especially Effective for Oligonucleotides
The repeating phosphate backbone of oligonucleotides gives rise to a strong and uniform anionic character across a wide pH range (typically pH 7–9). This makes oligonucleotides exceptionally well suited for anion exchange separations.
Key factors influencing AEX behavior include:
- Charge density: Longer oligonucleotides carry more negative charges and therefore exhibit stronger retention.
- Resin chemistry: Strong and weak anion exchangers differ in functional groups and pH stability, influencing selectivity and robustness.
- Buffer composition: The choice of buffering ions and salt gradients directly affects elution strength and resolution.
Salt gradients—commonly based on chloride ions—compete with the oligonucleotide backbone for binding sites on the resin. By carefully tuning gradient slope and composition, analysts can achieve highly reproducible and finely resolved separations.
 KNAUER: Oligos Made Easy - Part 2 AEX: Figure 3 Ionic interactions and pH influence on Anion Exchange Chromatography (AEX).
KNAUER: Oligos Made Easy - Part 2 AEX: Figure 3 Ionic interactions and pH influence on Anion Exchange Chromatography (AEX).
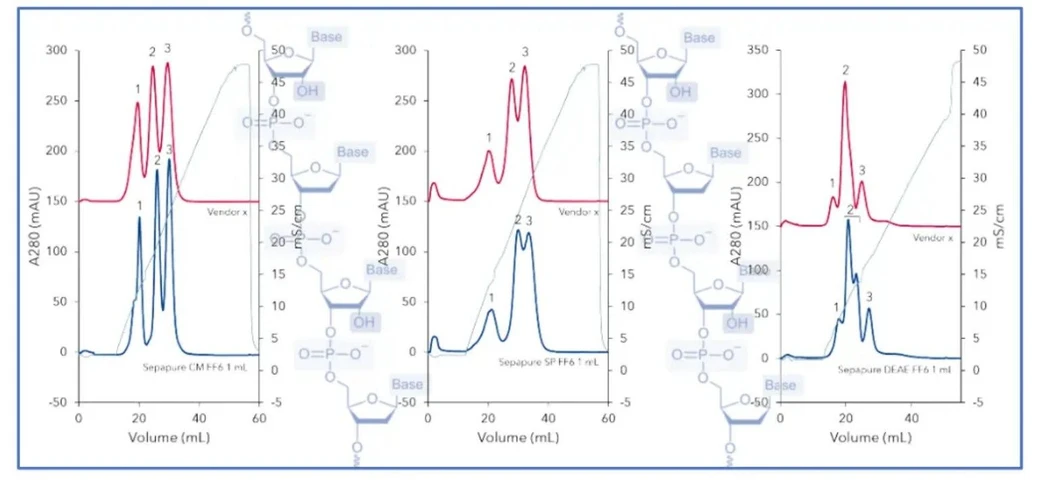
 KNAUER: Oligos Made Easy - Part 2 AEX: Figure 4 AEX buffer selection and gradient strategy (chromatogram with red buffer gradient curve).
KNAUER: Oligos Made Easy - Part 2 AEX: Figure 4 AEX buffer selection and gradient strategy (chromatogram with red buffer gradient curve).
AEX as the Gold Standard for Oligonucleotide Separation
Anion exchange chromatography has become the method of choice for both analytical and preparative oligonucleotide workflows. Its advantages include:
- High resolving power: Closely related impurities, including n–1 or n–2 species, can often be separated and quantified.
- Predictable retention behavior: In many cases, retention time correlates linearly with oligonucleotide length, simplifying identification.
- Compatibility with modified oligonucleotides: Even chemically modified sequences retain their anionic nature, allowing AEX to resolve both products and related impurities.
- Excellent control via gradient elution: Salt gradients provide precise tuning of selectivity and resolution.
Typically, shorter or degraded oligonucleotides elute at lower ionic strength, while full-length products with higher charge density appear later in the chromatogram.
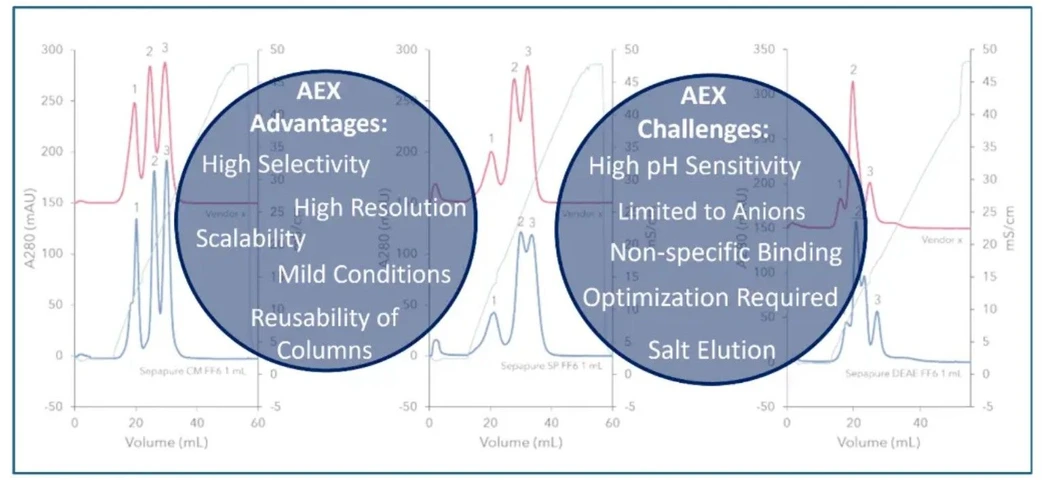 KNAUER: Oligos Made Easy - Part 2 AEX: Figure 5 AEX Advantages and Challenges.
KNAUER: Oligos Made Easy - Part 2 AEX: Figure 5 AEX Advantages and Challenges.
Final Perspective: Mastering AEX with Confidence
Anion exchange chromatography may appear complex at first, but with thoughtful optimization of pH, buffer composition, and gradient conditions, it becomes a highly precise and reliable tool for oligonucleotide analysis. When properly applied, AEX delivers sharp peaks, excellent resolution, and robust performance—even for challenging sequences and modifications.
For laboratories developing or refining oligonucleotide workflows, AEX offers both analytical depth and operational confidence.
If you would like to discuss AEX method development or optimization for oligonucleotides, feel free to contact us at [email protected]. More insights into practical oligonucleotide analysis will follow in our “Oligos Made Easy” series.
For technical questions or in-depth discussion, the author can be reached at [email protected]