Oligos Made Easy - Part 1: Overview

- Photo: KNAUER: Oligos Made Easy - Part 1: Overview
- Video: KnauerHPLC: KNAUER LNP Systems
A Practical Guide to Oligonucleotide Production – From Concept to Final Product
In 2020, an internal innovation at KNAUER significantly reshaped our perspective on nucleic acid technologies. The development of Impingement Jets Mixing (IJM) for lipid nanoparticle (LNP) generation—originally driven by the urgent need for mRNA-based COVID-19 vaccines—sparked a deeper engagement with DNA and RNA-based therapeutics. Although mRNA vaccines were the first application, it soon became clear that the same platform could support a broad range of oligonucleotides, including short DNA and RNA sequences.
This realization naturally expanded our focus beyond formulation alone. Purification strategies, analytical control, and scalable manufacturing of oligonucleotides became equally compelling challenges. Today, simplifying and optimizing oligonucleotide production workflows is a central pillar of KNAUER’s technology roadmap.
Technologies for LNP Manufacturing
- NanoScaler
- Customized NanoProducer
These systems provide flexible solutions for research and production-scale LNP generation, supporting nucleic acid delivery applications.
 KNAUER: Systems for LNP production. Middle - NanoScaler, Right - Customized NanoProducer
KNAUER: Systems for LNP production. Middle - NanoScaler, Right - Customized NanoProducer
What Is Required to Manufacture Oligonucleotides?
Whether producing oligonucleotides for internal use or as a CDMO, establishing a dedicated oligo production laboratory requires a sequence of well-defined steps. This introductory article outlines a mid-scale production workflow (from approximately 1 µmol up to several hundred mmol). Each operation is introduced here and will be explored in greater depth in future articles, with particular attention to purification and quality control. But before assessing purity, the oligonucleotide itself must first be synthesized.
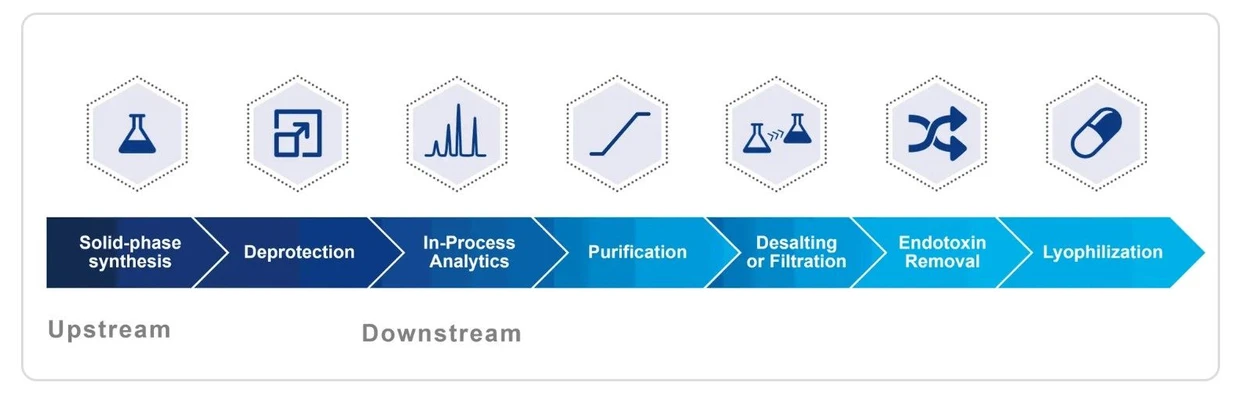 KNAUER: Typical workflow for oligonucleotide production
KNAUER: Typical workflow for oligonucleotide production
Typical Oligonucleotide Production Workflow
Step 1: Solid-Phase Oligonucleotide Synthesis
Oligonucleotide assembly begins on a solid support, most commonly controlled pore glass (CPG) or polystyrene resin. Individual nucleotides are sequentially added through a cyclic series of reactions:
- Detritylation – removal of temporary protecting groups
- Coupling – attachment of the next nucleotide
- Oxidation or thiolation – stabilization of the phosphate backbone
- Capping – deactivation of unreacted chains
These repetitive steps involve precise reagent delivery, washing, and activation, and are typically performed on automated synthesizers. KNAUER’s own oligonucleotide synthesizer is currently approaching completion.
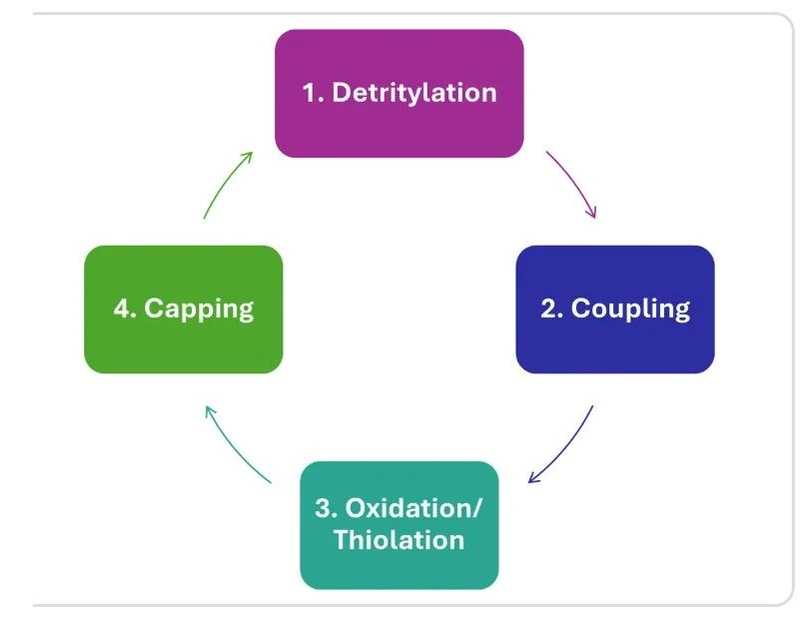 KNAUER: Solid-phase synthesis
KNAUER: Solid-phase synthesis
Step 2: Cleavage and Deprotection
Once synthesis is complete, the crude oligonucleotide must be released from the solid support. This is commonly achieved using ammonia-based reagents. Subsequent treatment with heated ammonium hydroxide removes remaining protecting groups. After filtration to remove the solid matrix, the solution is neutralized and prepared for initial analytical evaluation.
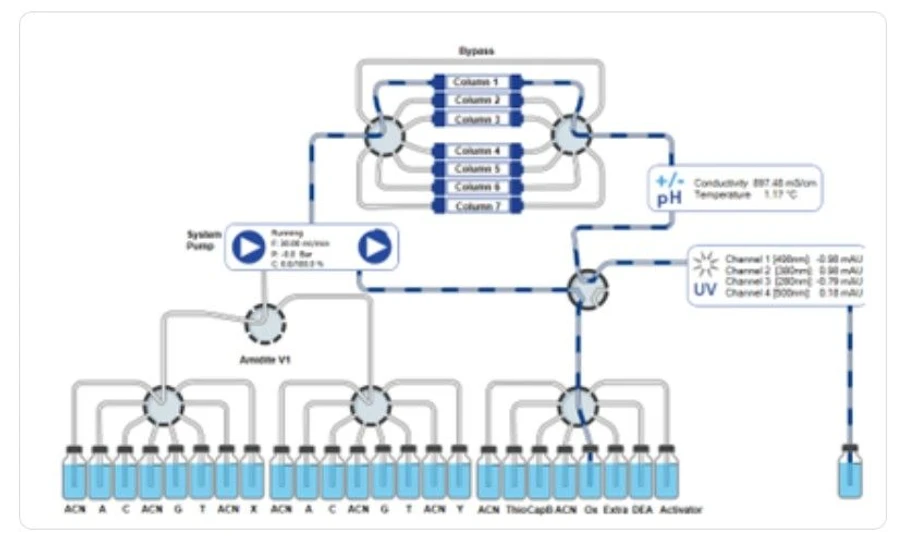 KNAUER: Flow scheme KNAUER Synthesizer
KNAUER: Flow scheme KNAUER Synthesizer
Step 3: Chromatographic Analysis and Purification
Chromatography plays a central role in both the characterization and purification of oligonucleotides. Commonly used techniques include:
- Anion-Exchange Chromatography (AEX)
Separation based on charge; cost-efficient but generates high salt loads requiring downstream removal. - Ion-Pair Reversed-Phase Chromatography (IP-RP)
Offers excellent resolution and typically simplifies downstream processing due to volatile buffers, though at higher operating costs. - Hydrophilic Interaction Liquid Chromatography (HILIC)
An increasingly popular analytical tool, particularly suitable for short or chemically modified oligos, without ion-pairing reagents. Its primary strength lies in analysis rather than large-scale purification.
Step 3a: In-Process Quality Control
Throughout production, oligonucleotides must be monitored for purity, sequence fidelity, and structural modifications. Liquid chromatography remains the primary analytical technique for these assessments, commonly using IP-RP, AEX, or HILIC methods. This is an area where KNAUER’s analytical expertise and HPLC platforms play a key role.
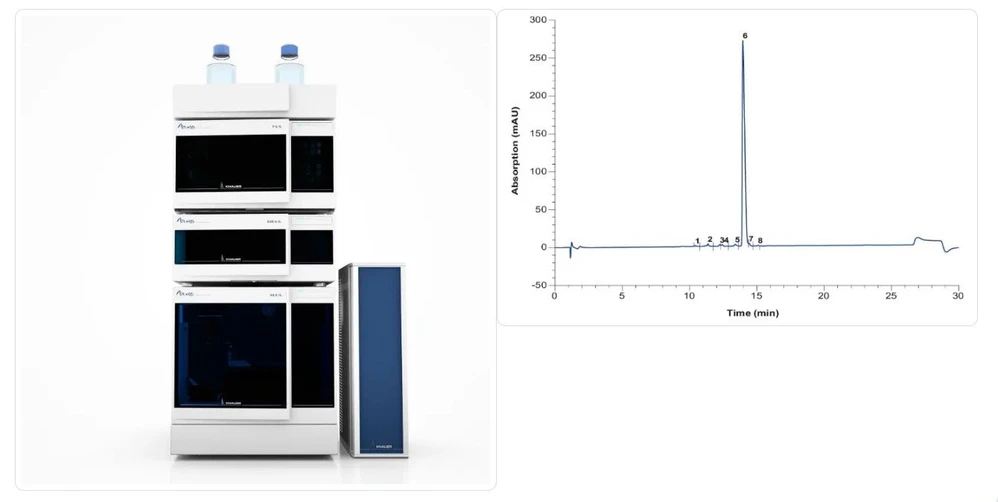 KNAUER: Representative KNAUER analytical HPLC system and Chromatogram of Oligonucleotide Analysis
KNAUER: Representative KNAUER analytical HPLC system and Chromatogram of Oligonucleotide Analysis
Step 3b: Preparative Chromatographic Purification
Following analytical assessment, purification removes synthesis-related impurities such as truncated sequences or residual protecting groups. AEX and IP-RP are the most frequently applied methods. Process development teams often evaluate both approaches at small scale before selecting the optimal strategy. Notably, both modes can be implemented on the same preparative system, such as the AZURA® Lab or Pilot Prep HPLC platforms.
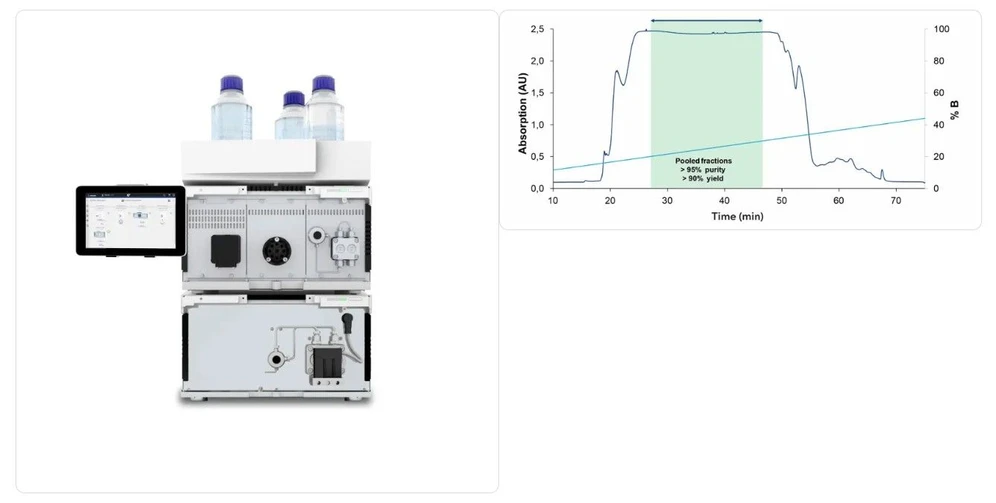 KNAUER: Representative KNAUER preparative LC System and Chromatogram of Oligonucleotide Purification
KNAUER: Representative KNAUER preparative LC System and Chromatogram of Oligonucleotide Purification
Step 4: Desalting or Solvent Exchange via Tangential Flow Filtration (TFF)
After chromatographic purification, oligonucleotides remain in matrices containing salts or organic solvents. Tangential flow filtration enables gentle and scalable buffer exchange. Low-molecular-weight components pass through the membrane, while oligonucleotides are retained. This step prepares the product for formulation or further processing. KNAUER is currently developing a modular TFF solution tailored to this application.
Step 5: Endotoxin Reduction
For therapeutic applications, endotoxin levels must be rigorously controlled. Lipopolysaccharides from bacterial sources can be efficiently reduced using an additional TFF step. By selecting appropriate membranes, multiple diafiltration cycles can lower endotoxin concentrations by several orders of magnitude, supporting regulatory compliance depending on dose and administration route.
Step 6: Lyophilization
For preclinical and early-stage batches, freeze-drying is the standard finishing step. The oligonucleotide solution is frozen below –40 °C and subjected to vacuum sublimation, producing a dry, stable product that can be stored at ambient temperature and rapidly reconstituted when needed.
Connecting the Workflow with KNAUER Technology
KNAUER provides a cohesive portfolio supporting oligonucleotide production from synthesis to final formulation. AZURA® (U)HPLC systems and dedicated columns such as Sepapure® oliGO enable robust analytical control and efficient purification. Preparative LC platforms are optimized for oligo workflows, while new solutions—including an in-house oligonucleotide synthesizer and a modular TFF system—are being developed to streamline desalting and endotoxin removal.
Together, these technologies allow laboratories to build scalable, integrated oligonucleotide production environments without relying on fragmented, multi-vendor solutions.




