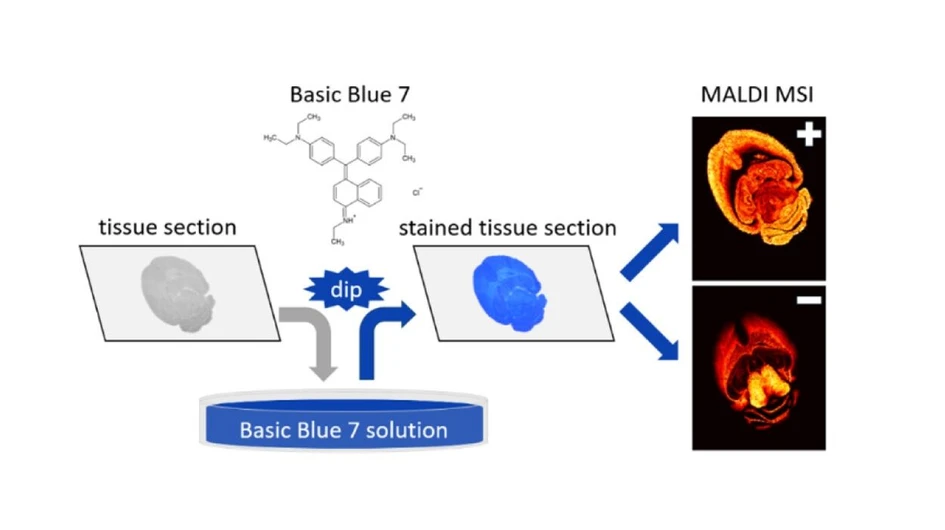
Staining Tissues with Basic Blue 7: A New Dual-Polarity Matrix for MALDI Mass Spectrometry Imaging
Anal. Chem. 2025, 97, 5, 2828–2836: Staining Tissues with Basic Blue 7: A New Dual-Polarity Matrix for MALDI Mass Spectrometry Imaging
This research aims to improve the reproducibility and quality of MALDI mass spectrometry imaging (MSI) by introducing Basic Blue 7 (BB7) as a new dual-polarity matrix. BB7, a triarylmethane dye with high water solubility, enables a straightforward sample preparation protocol using a dipping method instead of expensive spraying or sublimation techniques. This approach simplifies the workflow and makes the technique more accessible.
The study demonstrates BB7’s effectiveness in imaging lipids in mouse brain tissue, comparing its performance with traditional matrices like 2,5-dihydroxybenzoic acid (DHB) and 1,5-diaminonaphthalene (DAN). Particularly in negative ion mode, BB7 provides low background noise and high signal intensities across multiple lipid classes. Additionally, the stained tissue can be visually inspected for basic histopathological annotation before MSI analysis.
By combining efficient staining with mass spectrometry imaging, this method offers excellent image quality, reproducible sample preparation, and the potential for automation, making it a promising advancement for high-resolution MSI applications.
The original article
Staining Tissues with Basic Blue 7: A New Dual-Polarity Matrix for MALDI Mass Spectrometry Imaging
Michal Javorek, Michal Hendrych, Kateřina Ondráková, Jan Preisler, and Antonín Bednařík
Anal. Chem. 2025, 97, 5, 2828–2836
https://doi.org/10.1021/jasms.4c00455
licensed under CC-BY 4.0
Matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI MSI) is a powerful label-free technique for studying the spatial distribution of various biomolecules in biological tissues. (1) Despite the fast growth of this technique over the past decade, there are still many challenges in MALDI MSI that have not been completely resolved. (2) For example, complex spectra of commonly used matrices in low mass-to-charge (m/z) regions restraining the analysis of small molecules; (3) limited sensitivity for certain molecule classes; (2,4) reproducible sample preparation protocols (5) often requiring expensive spraying/sublimation equipment; (6,7) compatibility with other imaging and histology techniques; (8,9) and quantification (10) or increasing spatial resolution to micrometer ranges. (11) Although a universal MALDI matrix does not exist, many of these individual challenges can be addressed by selecting the proper matrix with suitable properties. (12)
A typical MALDI matrix is a small organic molecule that forms cocrystals with analytes, efficiently absorbs laser irradiation, and ionizes the analytes, primarily by proton transfer. (13) An ideal matrix produces simple MALDI mass spectra containing no or only a few signals originating from the matrix itself, to avoid interference with analyte signals. Furthermore, it should be able to provide quality spectra of multiple analytes in both positive and negative ion modes, i.e., be a so-called dual-polarity matrix. (14) Typically, a dual-polarity matrix has a high proton affinity and low deprotonation energy to produce both [M + H]+ and [M – H]− ions, which are provided by both acidic and basic functional groups incorporated into its structure. As there is no ideal or universal MALDI matrix for analysis and MSI of different classes of biomolecules (lipids, proteins, etc.), (12) different matrices are applied, and the quest for searching for novel matrices with better properties is a seemingly never-ending task. (15) Examples of recently introduced dual-polarity matrices for MALDI MSI are derivates of anthranilic acid, (14) cyanographene (16) or (E)-4-(2,5-dihydroxyphenyl)but-3-en-2-one. (17) Interesting classes of MALDI matrices are based on organic dyes, including fluorescent dyes, 2,3-dicyanohydroquinone, (13) IR-780, (18) or heterocyclic-based dyes. (19)
Lipids represent a widely studied group of biomolecules having a large diagnostic potential, thanks to the many essential functions they fulfill in an organism, ranging from cell membrane constitution to energy storage and molecular signaling. (20) With the variety of functions also comes a variety of lipid structures, which implies that not all classes of lipids ionize well in both positive/negative modes or using a single MALDI matrix. (21) Cholesterol (22) or phosphatidylcholines (PCs) are routinely analyzed in positive ion mode. (23,24) The negative mode is more suitable for the analysis of phosphatidylethanolamines (PEs), phosphatidylinositols (PIs), or ceramides. (25) Common matrices for successful MALDI MSI of lipids are 2,5-dihydroxybenzoic acid (DHB) or sinapinic acid (SA), (26) useful mostly in positive mode. In negative mode, 9-aminoacridine (9-AA) (27) or 2-(2-aminoethylamino)-5-nitropyridine (28) are most useful, and 1,5-diaminonaphthalene (DAN) in both polarities. (29)
Obtaining high-quality MS images and reproducibility of the technique depend on the sample preparation protocol. Perhaps the most crucial part is the application of the MALDI matrix. (30) With increasing MALDI MSI spatial resolution to the micrometer range, the demands for matrix application also increase in terms of better homogeneity of the matrix layer and smaller size of produced crystals. (31) In addition, preserving the structural integrity and preventing analyte delocalization during sample preparation is essential. (32) Currently, two approaches dominate sample preparation for MALDI MSI: pneumatic spraying and sublimation. Spraying can be a relatively fast and simple method to create a homogeneous matrix layer. (33) Depending on the parameters of the spraying, a wet or dry aerosol is generated. Wet spray can increase the extraction of analytes from tissues and lead to augmented signals. However, an excessive amount of wet matrix solution can lead to the delocalization of analytes. (34) On the other hand, sublimation is a dry method that efficiently prevents the delocalization of analyzed molecules. (35) After matrix sublimation, recrystallization is recommended for additional extraction of analytes from the tissue into the matrix layer, increasing signals up to an order of magnitude. (36) More specialized methods of matrix application, such as dry coatings (37,38) or incorporation of the matrix in agar medium, have been proposed. (39) Tissue staining with dyes traditionally used for histological staining of lipids, namely Sudan Black B, Oil Red O, and Nile Blue A, has also been investigated. (19) However, for successful MSI using these dyes, previous lipid insolubilization with a chromate solution or chemical fixation with osmium tetroxide is necessary.
In this work, we present a new dual-polarity matrix for MALDI MSI: Basic Blue 7 (BB7), with the systematic name N-(4-((4-(diethylamino)phenyl)(4-(ethylamino)naphthalen-1-yl)methylene)cyclohexa-2,5-dien-1-ylidene)-N-ethylethanaminium chloride (Figure 1A). BB7 belongs to the group of triarylmethane dyes. (40) It is often used to dye paper, wool, silk, nylon, or as an ink. (41) Owing to adequate solubility in water (∼20 mg·mL–1), this matrix allows a simple sample preparation protocol resembling the staining methods of classical histopathology without previous chemical fixation of the tissues: dipping the glass with mounted tissue into the dye solution. Tissue staining with BB7 is explored in detail, and its MALDI MSI performance is compared with traditional sample preparation protocols using DHB and DAN matrices.
 Anal. Chem. 2025, 97, 5, 2828–2836 - Figure 1. A) Structure and B) UV–vis absorption spectrum of BB7..jpeg
Anal. Chem. 2025, 97, 5, 2828–2836 - Figure 1. A) Structure and B) UV–vis absorption spectrum of BB7..jpegExperimental Section
Preparation of Tissue Sections
Brains from BALB/c mice, frozen in liquid nitrogen immediately after surgical extraction, were stored at −80 °C. Sections with a 10 μm thickness were cut using a cryostat microtome CM1850 (Leica Microsystems, Germany) at −20 °C and thaw mounted onto standard microscopic glass slides. A series of sections from the same brain was stained with BB7 or covered with classical matrices by sublimation in a glass sublimation chamber (model GPE-1207–030PS, GPE Scientific, United Kingdom). An amount of 200 mg of DHB or DAN was homogeneously spread in the bottom part of the sublimation chamber. The sublimation parameters were 135 °C, pressure ≤ 100 mTorr, 4 min 20 s for DHB. DAN was sublimated at 130 °C, pressure ≤ 100 mTorr for 2 min 20 s.
BB7 Staining
For BB7 staining, 100 mg of BB7 (dye content 90%) was dissolved in 10 mL of deionized water. The solution was sonicated for 5 min in an ultrasonic bath and decanted prior to staining to remove residual undissolved dye particles. Subsequently, the glass slide with the tissue section was immersed 3 times for 10 s and 3 times for 30 s, for positive and negative mode measurements, respectively. There were 5 s intervals between each immersion. Finally, the glass with the stained tissue section was washed in a beaker with deionized water and dried under a gentle stream of dry air. The BB7 solution could be used for staining multiple tissues. After processing, the used BB7 solution was collected in a waste container. As BB7 can cause serious eye damage, all manipulation with this compound was done by wearing protective gloves and goggles.
BB7 Spraying
The BB7 solution was prepared by dissolving 5 mg of the dye powder (dye content 90%) in 1 mL of deionized water. An amount of 300 μL of the solution was pipetted into the airbrush pistol (Grafo T1, 0.15 mm, HARDER and STEENBECK, Germany) reservoir and was manually sprayed onto the sample from a distance of 14 cm. The spraying was realized in cycles: approximately 1 s of spraying was followed by 2 s of drying until the solution was consumed. The solution flow was 150 μL·min–1.
MALDI MSI
MALDI MS and MSI data were acquired using an orbital trap (Q Exactive Plus Orbitrap, Thermo Fisher Scientific, Germany) equipped with a subatmospheric (subAP) MALDI source (SubAP/MALDI(ng), MassTech, Inc., USA). Ions were transferred from the source to the MS by a two-stage ion funnel. Voltages V7–V1 applied across the funnel were 350, 270, 150, 120, 90, 85, 10 V and −250, −150, −120, −100, −90, −85, and −10 V in positive and negative modes, respectively. The radio frequency (RF) amplitude confining the ions passing through the ion funnel was 130 V (peak-to-peak). Pressure in the source was kept at 2 Torr, providing the optimal signal of phospholipids and ceramides at m/z range of 600–900. The mass resolving power was 140,000 (at m/z 200) in all experiments. If not stated otherwise, the pixel size was set to 50 μm.
A sample was continuously moved at a speed of 3.445 mm·min–1 under the stationary frequency-tripled Nd:YAG laser (355 nm, 1000 Hz) with a nearly circular spot with a diameter of ∼15 μm (based on microscopic inspection of ablated spots in a DHB layer). In the positive mode, the laser energy was set to 0.32 and 0.60 μJ·pulse–1 for DHB and BB7, respectively. For DAN and BB7 in negative ion mode, 0.36 μJ·pulse–1 and 0.90 μJ·pulse–1 were used, respectively. The m/z calibration in positive and negative modes was done using red phosphorus. (43)
Lipid Identification
The lipids were identified based on the exact measured mass, which was compared to the Lipid Maps database. The cases with measured m/z error less than 0.001 were considered positive assignments.
 Figure 4. (A) Photo of the BB7-stained mouse brain. MALDI MSI of lipid PC 40-6 recorded from (B) the same BB7-stained section and (C) parallel section with the sublimated DHB layer in positive ion mode..jpeg
Figure 4. (A) Photo of the BB7-stained mouse brain. MALDI MSI of lipid PC 40-6 recorded from (B) the same BB7-stained section and (C) parallel section with the sublimated DHB layer in positive ion mode..jpeg
Conclusions
We have introduced a new dye-based MALDI MSI matrix, Basic Blue 7, which is soluble in water and thus can easily be used for staining biological tissues, similar to methods used in classical histology. This opens a new and extremely simple method for MALDI MSI sample preparation. The staining technique does not require any special instrumentation and can be easily adapted and automated, which is a crucial factor in clinical practice. The BB7 staining is usable in positive ion mode, where alkali salts are efficiently washed out of the tissue, leading to a dominant presence of [M + H]+ ions. The developed protocol excels especially in the generation of negative ions by proton transfer from the analyte to the matrix, thanks to the basic tertiary amide groups in the molecule. Obtained molecular maps of BB7-stained tissues are very sharp, comparable to maps obtained using sublimated DHB and DAN, without any signs of lipid delocalization or spreading out of the tissue borders. The staining protocol has great potential in high-spatial-resolution imaging, as BB7 is adsorbed on the tissue during staining with no visible crystals, the size of which often limits the spatial resolution in MALDI MSI. A certain limitation of the staining protocol is that it cannot be used for the imaging of water-soluble analytes. Compared to DAN or DHB, BB7 is a relatively hot matrix, inducing in-source fragmentation of certain lipids, and it requires higher laser pulse energy for optimal results. In contrast to DAN, which is a potential carcinogen, working with BB7 is generally safe. However, it is necessary to wear protective goggles as it can cause serious eye damage. Also, the extent of ion suppression effects caused by a variation in the amount of BB7 in the different tissue regions remains to be further characterized. On the other hand, due to the intense blue color, the stained tissues can be easily inspected and even used for direct histology annotation, which simplifies the comparison of ion images with the various tissue regions. Last but not least, there is a wide variety of other triarylmethane dyes to which BB7 belongs, and many of these dyes can be expected to perform in MALDI MSI in a similar way or even better. In the future, this approach can present a shared point between classical and modern histology techniques and facilitate the spreading of MALDI MSI into clinical practice.