Online SEC-UV-RP-MS for characterization of adeno-associated viruses (Megane Aebischer, MDCW 2025)
- Photo: MDCW: Online SEC-UV-RP-MS for characterization of adeno-associated viruses (Megane Aebischer, MDCW 2025)
- Video: LabRulez: Megane Aebischer: Online SEC-UV-RP-MS for characterization of adeno-associated viruses (MDCW 2025)
🎤 Presenter: Megane Aebischer (University of Geneva, Geneva, Switzerland)
💡 Book in your calendar: 17th Multidimensional Chromatography Workshop (MDCW) 13 - 15. January 2026
Abstract
Adeno-associated virus (AAV) vectors have become one of the preferred choices for gene therapies, with several FDA-approved products and numerous clinical trials underway. This preference is attributed to AAV's broad tissue tropism, non-pathogenic nature, favorable safety profile, and ability to sustain long-term transgene expression.
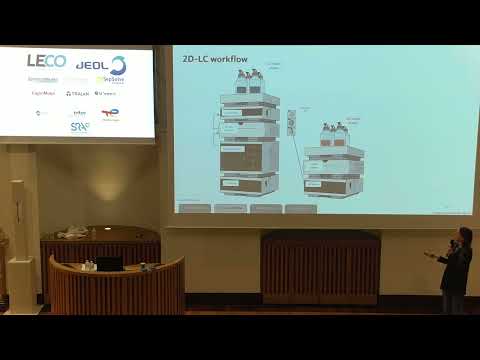
Despite their relatively simple structure, AAV-based gene therapy products pose challenges for their characterization due to large size and heterogeneity. Various chromatographic methods are classically employed to measure critical quality attributes (CQAs), such as aggregation levels, viral protein composition, and post-translational modifications (PTMs). To address the industry's need for more efficient characterization methods, multidimensional liquid chromatography (2D-LC) techniques have been developed to analyze multiple CQAs in a single run.
In this study, we present a novel SEC-UV-RP-MS method for simultaneous measurement of high molecular weight species (HMWs), viral protein ratio, capsid identity, and PTMs. This simple 2D-LC-MS approach integrates size exclusion chromatography (SEC) for aggregate semi-quantification in the first dimension, with reversed-phase liquid chromatography (RP-LC) coupled to mass spectrometry (MS) for detailed analysis of viral proteins in the second dimension. The developed collecting interface is relatively simple and the 2D-LC method was successfully applied for the comprehensive, streamlined, and versatile characterization of several AAV serotypes (AAV2, AAV8 and AAV9). Forced degradation study was performed using this generic method to demonstrate the platform’s sensitivity in detecting changes in AAV stability, such as increased aggregation and deamidation at elevated temperatures. This 2D-LC-MS approach confirms the applicability of multidimensional liquid chromatography for AAV vector characterization, providing a powerful tool for comprehensive analysis of gene therapies vectors.
-Workshop-LOGO_s.webp)



