ANALYTIKA - 20% discount on Domifen bromide

ANALYTIKA: ANALYTIKA - 20% discount on Domifen bromide
Domifen bromide - Certified reference material for cosmetic and pharmaceutical research
Specification
- Product Code: DRE-C13081000
- CAS No.: 538-71-6
- Mol. Weight: 414.46
- Mol. Formula: C22H40NO · Br
- Storage Temp: 4°C ± 4°C
- Expiry Date: 30 Jun 2028
Certified
- Purity: 99.22% (g/g)
- Expanded Uncertainty (U): 0.30% (g/g)
Uncertainty
The certified value(s) and uncertainty(ies) are determined in accordance with ISO 17034 with an 95% confidence level (k=2). Uncertainty is based on the Total Combined Uncertainty, including uncertainties of characterisation, homogeneity and stability testing. Stability values are based on real evidence opposed to simulation.
Characterisation
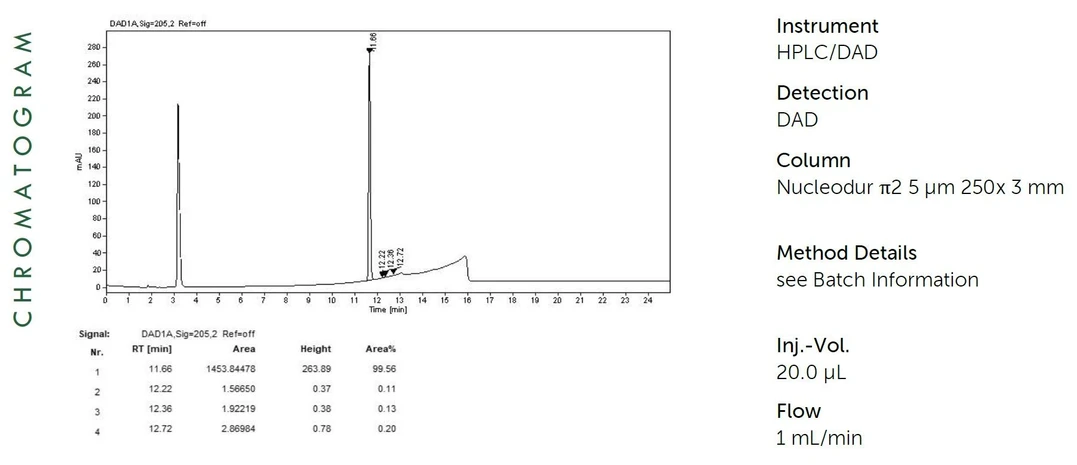 ANALYTIKA: HPLC Characterisation of Domifen bromide
ANALYTIKA: HPLC Characterisation of Domifen bromide
Method of Characterisation
- Purity = 100% - Assay impurities – Water content (KF)
Method of Identification
- EA, NMR, RT, IR, MS, UV
Batch Information
- Water Content: 0.27% (g/g) by Karl-Fischer-Titration (U(exp) = 0.04% (g/g)).
Method Details
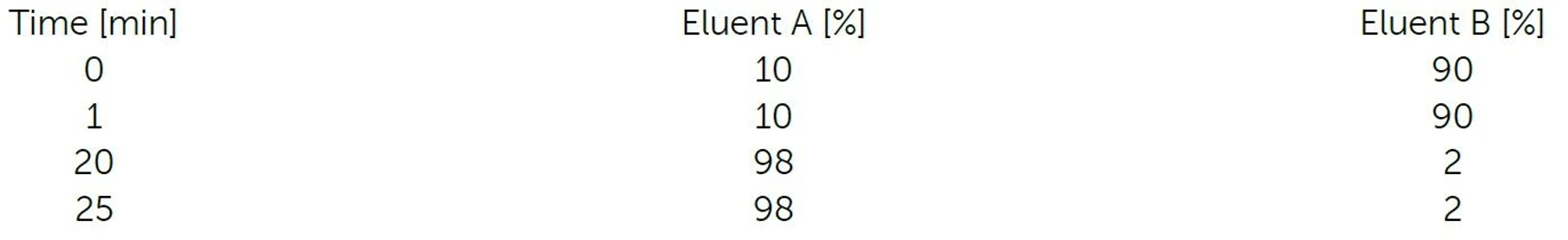
- Eluent A: 100% Acetonitrile
- Eluent B: Water + H3PO4
 ANALYTIKA: HPLC Characterisation of Domifen bromide
ANALYTIKA: HPLC Characterisation of Domifen bromide
Intended Use
This CRM is intended for use in a laboratory as a calibration and quality control standard or in method development for analytical techniques.
Safety
Proper precautions should be observed while handling. See Safety Data Sheet.
Traceability
The balances used for gravimetric measurements are calibrated with weights traceable to the national standards (DKD). The calibration of the balances is verified daily internally and annually by an external accredited calibration service. Chromatographic methods are traceable to the International System of Units (SI).
Homogeneity
Random replicate samples of the final packaged CRM have been analyzed to prove homogeneity compliant with ISO 17034.
Storage
The CRM should be stored in the original sealed container at the indicated temperature.
Instructions for Use
It is recommended to use 1 mg as the minimum sample size, and if less material is used, to increase the certified uncertainty by a factor of two for half sample and four for a quarter of sample. If storage after opening is necessary, the CRM should be tightly closed and kept from light and moisture. If the CRM was in a sealed ampoule, it should be transferred to a vial with minimum headspace.
Visit the support section of our webiste for a series of Dr. Ehrenstorfer Tech Tip videos and frequently asked questions.




