Gunacins: Novel Benzo[g]chromene Derivatives from the Fungus Exobasidium sp. and Their Potent Anti-Leishmania and Trypanosoma Activities
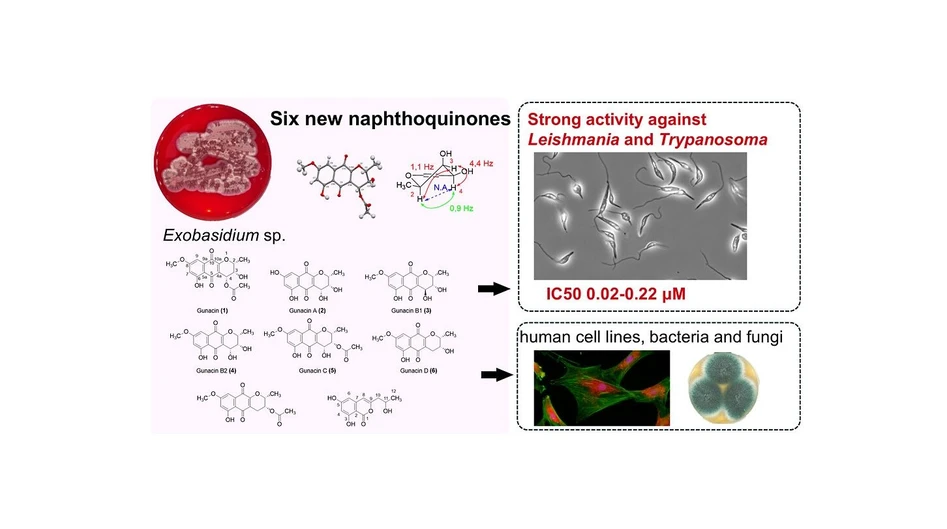
ACS Omega 2025, 10, 22, 23222–23234: Graphical abstract
Six new pyranonaphthoquinone derivatives, gunacin A–E, together with known gunacin and the isocoumarin (+)-orthosporin, were isolated from the fungus Exobasidium sp. Their structures were elucidated using X-ray crystallography, detailed NMR analysis including ROESY, and mass spectrometry. Two compounds showed remarkable potency against Leishmania mexicana and multiple Trypanosoma species, with EC₅₀ values as low as 0.02–0.24 μM—on par with clinically used antiparasitic drugs.
In addition to their antiprotozoal effects, several gunacins demonstrated antibacterial and moderate antifungal activity, and exhibited a broad range of cytotoxicity toward mammalian cell lines. This study is one of the first to explore the chemical potential of Exobasidium, a genus of phytopathogenic Basidiomycota fungi, and highlights smut fungi as an underexplored source of bioactive natural products. The potent activity of gunacins suggests promising opportunities for developing new antiprotozoal therapeutics.
The original article
Gunacins: Novel Benzo[g]chromene Derivatives from the Fungus Exobasidium sp. and Their Potent Anti-Leishmania and Trypanosoma Activities
Eva Stodůlková, Dominik Lovás, Miroslav Flieger, Alena Zíková, Jakub Zápal, Martin Štícha, Ivana Císařová, Jan Černý, Valéria Grobárová, Martina Slapničková, Tomáš Vomastek, Zuzana Klímová, Marek Kuzma, Jaroslav Semerád, Tomáš Cajthaml, Eva Cséfalvay, Winnie Cherotich Maritim, Adéla Wennrich, Marc Stadler, Tereza Ježková, Andrej Jašica, and Miroslav Kolařík*
ACS Omega 2025, 10, 22, 23222–23234
https://doi.org/10.1021/acsomega.5c01325
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Pyranonaphthoquinones are a diverse and widespread group of secondary metabolites found in plants, (1,2) fungi, (3,4) and bacteria (5,6) with the majority possessing the 3,4-dihydro-1H-benzo[g]chromene-5,10-dione structural motif. Only a few pyranonaphthoquinones feature the 3,4-dihydro-2H-benzo[g]chromene-5,10-dione skeleton, including α-lapachone, constituents of “Lapacho tea”, used in traditional herbal medicine and believed to have anticancer effects. (7,8) Other examples include rhinacanthin A and its derivatives from plant Rhinacanthus nasutus, (9) α-caryopterone from Caryopteris clandonensis, (10) and gunacin (1) isolated from the fungus Ustilago sp. (3) Lapachones and rhinacanthins exhibit a wide range of biological activities, including cytotoxic, (9,11) antibacterial, (1,12) antifungal (13,14) and antiprotozoal effects. (1)
Exobasidium species (15) (Exobasidiales, Ustilaginomycotina, Basidiomycota) are worldwide distributed biotrophic plant pathogens, almost infesting plants within the Ericales order. (16) The genus Exobasidium causes notable economic losses in tea and blueberry production. (17,18) Despite their prevalence and importance, there have been very few studies on secondary metabolites produced by Exobasidium species. Moreover, a study describing the isolation of scopoletin and scopolin fails to distinguish whether the secondary metabolites originate from the plant host or the fungus itself. (19) The only credible publications have described the production of auxins, 2-(1H-indol-3-yl) acetic acid, ethyl 2-(1H-indol-3-yl) acetate, (20) and (S)-2-hydroxy-3-phenylpropanoic acid, (21) by axenic Exobasidium cultures.
This study represents the first comprehensive analysis of Exobasidium secondary metabolites, their isolation, structure determination, and biological activities.
2. Results and Discussion
2.1. Structure Elucidation
In this study, we identified six new pyranonaphthoquinone derivatives, gunacin A–E (2–7), along with the known compounds gunacin (1) and the isocoumarin derivative (+) orthosporin (8) (Figure 1). Gunacin (1) (2R,3S,4R)-3,6-dihydroxy-8-methoxy-2-methyl-5,10-dioxo-3,4,5,10-tetrahydro-2H-benzo[g]chromen-4-yl acetate was obtained as orange-red needles which crystallized from the MeOH/CH2Cl2 mixture. With the molecular formula C17H16O8, determined by positive high-resolution electrospray ionization mass spectrometry (HRESIMS), data showed a protonated ion [M + H]+ at m/z 349.0920 (calcd for C17H16O8+, 349.0918).
 ACS Omega 2025, 10, 22, 23222–23234: Figure 1. List of isolated compounds.
ACS Omega 2025, 10, 22, 23222–23234: Figure 1. List of isolated compounds.
2.2. Biological Activity
The antimicrobial activity of compounds 1, 3, 5, 6, and 7 was evaluated against model microorganisms, including Escherichia coli, Kocuria rhizophila, Cryptococcus neoformans, and Candida albicans. All tested compounds demonstrated better inhibitory activity against bacteria compared with the positive control, chloramphenicol. The activity against yeasts was approximately on par with the positive control, cycloheximide, except for 3, which showed no inhibitory activity even at the highest tested concentration of 66.7 μM (Table 5).
 ACS Omega 2025, 10, 22, 23222–23234: Table 5. Minimal Inhibition Concentration (MIC) of Compounds Gunacin (1), Gunacin A (2), Gunacin B1 (3), Gunacin B2 (4), Gunacin C (5), Gunacin D (6), and Gunacin E (7) against Model Pathogenic Microorganisms E. coli, K. rhizophila, C. neoformans, and C. albicansa
ACS Omega 2025, 10, 22, 23222–23234: Table 5. Minimal Inhibition Concentration (MIC) of Compounds Gunacin (1), Gunacin A (2), Gunacin B1 (3), Gunacin B2 (4), Gunacin C (5), Gunacin D (6), and Gunacin E (7) against Model Pathogenic Microorganisms E. coli, K. rhizophila, C. neoformans, and C. albicansa
In terms of the morphological effects, HeLa cells and human fibroblasts were examined. The efficacy of 5 on primary fibroblasts is evidenced by the complete disappearance of the MitoTracker Red CMXRos mitochondrial signal at a concentration of 5 μM, which is indicative of mitochondrial dysfunction, particularly with regard to the mitochondrial membrane potential. At higher concentrations, the cells lose their adherence to the surface. At lower concentrations, the actin cytoskeleton remains unaffected, showing the compound’s selectivity for mitochondria. In contrast, HeLa cells are more resistant, with effects on mitochondrial physiology only observed at the highest tested concentration of 125 μM, while the actin skeleton remains unchanged. Compound 1 has similar activity as 5, HeLa cells are significantly more resistant, with notable alterations in cellular morphology only at 125 μM. Effects on fibroblasts were observed at 5 μM, including a loss of polymerized actin and mitochondrial signal. Compound 7 did not show significant toxicity even at 125 μM on Hela cells, except for a slight decrease in the green signal for actin. The concentration 25 μM did not show any notable effect on fibroblast cell line but concentration 125 μM caused complete loss of live adherent cells. Compound 3 was more potent against HeLa cells, showing a weaker morphological effect at 25 μM but caused complete loss of live adherent cells at 125 μM, with only a weak effect on mitochondrial signal in fibroblast cells at 125 μM (Figure 4).
 ACS Omega 2025, 10, 22, 23222–23234: Figure 4. Visualization of the effects of compound 1, 3, 5, 7 on the morphology of HeLa adenocarcinoma cell line (left) and primary human skin fibroblasts (right). DMSO was used as a solvent and as a control. Mitochondria are visualized using MitoTracker Red CMXRos (red), the actin cytoskeleton with Phalloidin (green), and nuclei with DAPI (blue).
ACS Omega 2025, 10, 22, 23222–23234: Figure 4. Visualization of the effects of compound 1, 3, 5, 7 on the morphology of HeLa adenocarcinoma cell line (left) and primary human skin fibroblasts (right). DMSO was used as a solvent and as a control. Mitochondria are visualized using MitoTracker Red CMXRos (red), the actin cytoskeleton with Phalloidin (green), and nuclei with DAPI (blue).
Our study is also one of the few focusing on metabolites from smut fungi (specifically the order Exobasidiales), demonstrating that these overlooked fungi are potent producers of secondary metabolites. Little is known about the ecological role of the compounds they produce. The species we studied is not known to develop a pathogenic phase, which is otherwise typical for all members of the genus Exobasidium. Thus, it remains unclear whether these metabolites are involved in plant interactions. Instead, they might play a role in microbial competition within their environment.
3. Experimental Procedures
3.1. General Experimental Procedures
Optical rotations were determined using a Rudolph Research Analytical Autopol III automatic polarimeter in MeOH or acetone. NMR spectra were obtained using a Bruker Avance III 700 MHz spectrometer (700 MHz for 1H, 176 MHz for 13C) and a Bruker Avance III 600 MHz spectrometer (600 MHz for 1H, 151 MHz for 13C). All samples were measured in CD2Cl2. The spectra were referenced by the residual signal of the solvent (CD2Cl2: δH 5.323 ppm, δC 53.87 ppm). HRESIMS analysis and tandem mass spectrometry were performed on a Bruker qTOF Compact instrument with Agilent 1290 Infinity II high-performance liquid chromatography (HPLC) instrument equipped with Kinetex biphenyl columns (100 × 2.1; 100 Å). Semipreparative HPLC was carried out on a Waters instrument equipped with a 2487 UV detector and Gemini C18 column (5 μm, 110 Å, 250 × 10.00 mm). Column chromatography (CC) was performed on Sephadex LH-20 (150 g, GE Healthcare Bio-Science, Sweden), STRATA C18 column (20 g, 55 μm, 70 Å Phenomenex); silica gel (0.035–0.070 mm, 70 Å, Lachner) fractions were analyzed by high-performance liquid chromatography (HPLC) with UV–vis detection using an Alliance HPLC 2988 dual photodiode array (PDA) detector equipped with a Gemini C18 analytical column (5 μm, 110 Å, 250 × 4.6 mm). The melting point was determined using CNYST micromelting point measuring instrument and are uncorrected.