Quantification of Endogenous Steroids and Hormonal Contraceptives in Human Plasma via Surrogate Calibration and UHPLC-MS/MS
Anal. Chem. 2025, 97, 25, 13496–13503: Graphical abstract
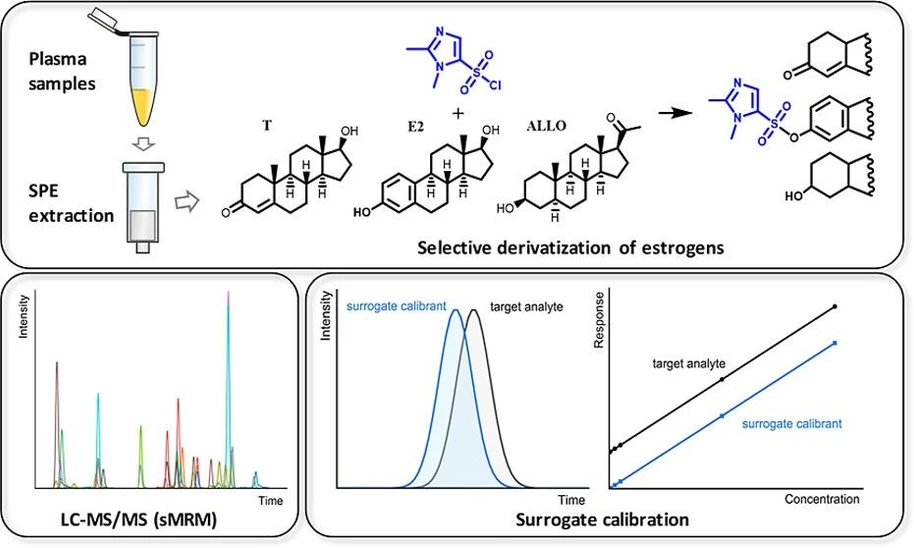
Quantifying endogenous and exogenous steroids in biological matrices is challenging due to low concentrations and cross-reactivity in immunoassays. To overcome the lack of a true blank matrix for calibration, we developed a surrogate calibration UHPLC-MS/MS method using 1,2-dimethylimidazole-5-sulfonyl chloride (DMIS) derivatization for estrogens, enabling sensitive quantification alongside nonderivatized steroids. Stable isotope-labeled surrogate calibrants and internal standards ensured matrix-matched accuracy, with parallelism confirmed across multiple levels in plasma.
The method combines narrow-bore UHPLC columns, optimized chromatographic conditions, protein precipitation, and 96-well SPE to achieve pg/mL-level sensitivity. It uniquely integrates surrogate calibration for endogenous steroids with external calibration for exogenous contraceptives, delivering comprehensive hormonal profiling in a single run. Its validation strategy aligns with regulatory guidelines, offering a practical reference for future LC–MS/MS assays using surrogate calibration.
The original article
Quantification of Endogenous Steroids and Hormonal Contraceptives in Human Plasma via Surrogate Calibration and UHPLC-MS/MS
Min Su, Bernhard Drotleff*, Tamara Janker, Zoé Bürger, Ann-Christin S. Kimmig, Birgit Derntl, Michael Lämmerhofer
Anal. Chem. 2025, 97, 25, 13496–13503
https://doi.org/10.1021/acs.analchem.5c01912
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
LC–MS/MS is a well-established technique for steroid analysis, valued for its fast analysis time, high specificity, minimal sample volume, and broad analyte coverage within a single injection. Steroid separation is typically performed using reversed-phase (RP) columns with C8, C18, or phenyl-modified silica stationary phases. (9−12) To enhance separation efficiency, improve sensitivity, and shorten analysis time, ultrahigh-performance liquid chromatography (UHPLC) with sub-2 μm particle columns and core–shell particle technology have been increasingly utilized. (13,14) An additional option to enhance sensitivity for challenging applications is the usage of columns with a narrow internal diameter (ID), e.g. 1.0 mm ID columns. (15) Besides increased sensitivity due to higher analyte concentration during detection and improved ionization efficiency, 1.0 mm ID columns also drastically reduce solvent consumption by requiring lower flow rates. However, they present challenges such as limited loading capacity and susceptibility to extra-column band broadening, requiring careful system optimization to fully leverage their benefits.
Triple-quadrupole mass spectrometers, which are classically operated in multiple-reaction monitoring (MRM) mode to simultaneously record selective precursor-to-fragment transitions, have been extensively utilized in the quantitative analysis of steroids by LC–MS/MS. (11,16,17) Furthermore, the application of scheduled MRM (sMRM) mode allows for the detection of a broader range of analytes with maximum dwell time in a single analytical run while maintaining at least 12 data points per peak of interest, ensuring reliable quantification without compromising sensitivity.
Absolute quantification of endogenous compounds, such as steroids in plasma, using LC–MS/MS remains challenging due to the absence of truly analyte-free biological matrices. Several strategies have been adopted to address this issue. (18−20) Surrogate calibration was selected for this study as it enables precise and accurate quantification in true matrix with distinct benefits compared to alternatives like standard addition, background subtraction, and surrogate matrix approaches. (12,21−25) Background subtraction is prone to inaccuracies, especially when quantifying concentrations below background level, while standard addition is time-consuming, involves extrapolation (with susceptibility to large variance caused by outliers), and requires larger sample volumes. In contrast, surrogate calibration is the most robust to control matrix effects and the only approach that allows reliable determination of LODs, LOQs and linear ranges of target analytes in the matrix. By spiking stable-isotope-labeled analogue (SIL) into the true matrix, the surrogate calibrants (often termed surrogate analytes in the literature) closely mimic the behavior of the target analytes. After initial response matching of the SIL to target analytes, and verification of parallelism, the concentration of the endogenous analyte is determined using the regression equation derived from the surrogate SIL calibration curve. (26,27)
The most commonly used extraction techniques for steroid analysis include protein precipitation (PP), (28,29) liquid–liquid extraction (LLE), (30−32) 96-well plate supported liquid extraction (SLE), (33) and solid-phase extraction (SPE). (12,34,35) Also, a one-step sample preparation combining PP with phospholipid removal by off-line SPE (with zirconia metal oxide-coated silica) achieved adequate performance for steroid analysis in clinical laboratories. (36)
Precolumn derivatization is another widely used technique to improve the sensitivity, (37−39) particularly for quantifying estrogens in e.g. plasma from female administering hormonal contraceptives, where endogenous estrogen levels are markedly suppressed. As reported, derivatization with reagents such as dansyl chloride, (10,40,41) hydroxylamine, (42) picolinoyl chloride hydrochloride, (43) or p-toluenesulfonylhydrazide (44) introduces additional functional groups to either phenol or keto moieties of estrogens, enhancing ionization efficiency and altering fragmentation patterns and chromatographic retention.
In the present study, we developed an LC–MS/MS-based method for the analysis of endogenous and exogenous hormones in plasma. 1,2-Dimethylimidazole-5-sulfonyl chloride (DMIS) (45,46) was used for the selective derivatization of estrogens. It showed improved sensitivity and specificity due to its estrogen-specific fragmentation. To ensure accurate and precise quantification, 13C-labeled and deuterated SIL analogues were used as surrogate calibrants and internal standards, respectively. In order to maximize sensitivity for the challenging target analyte class, we combined PP and SPE for analyte purification, followed by concentration through evaporation and low-volume reconstitution. Furthermore, the method incorporated the usage of 1.0 mm ID columns and selective DMIS derivatization of estrogens, allowing for pg/mL-level quantification of 12 endogenous and 5 exogenous hormones by simultaneously detecting derivatized estrogens and nonderivatized steroids. This work demonstrates that surrogate calibration can be effectively applied to a large panel of targets simultaneously and is the first to showcase its feasibility in combination with derivatization. Considering the multiple challenges in steroid quantification of endogenous and exogeneous steroids, optimization of a number of analytical details (SPE, derivatization, narrow-bore column, surrogate calibration) has led to an optimized robust steroid assay.
Experimental Section
LC–MS Method
The chromatographic instrumentation consisted of a 1290 Infinity II LC and Multisampler system (G7120A and G7167B, Agilent Technologies). Separation was performed on a Kinetex XB-C18 column (100 mm × 1.0 mm, 2.6 μm, 100 Å pore size) with a KrudKatcher Ultra in-line filter for column protection (both Phenomenex, Torrance, CA, USA). The mobile phase was delivered at a flow of 0.1 mL/min. A column temperature of 30 °C was maintained in an external column oven (MicroLC 200 oven, Sciex, Concord, Ontario, Canada), which was installed in close proximity to the ion source inlet to reduce extra-column volume. Mass spectrometric detection was performed on a QTRAP 4500 mass spectrometer with a Turbo V electrospray ionization (ESI) source (Sciex). Further details about LC–MS settings are provided in the Supporting Information and summarized in Table S1. The analytical system was controlled by the Analyst 1.7.1 software (including Analyst Device Driver 1.3, Sciex).
Results and Discussion
LC–MS Method
Considering the lower flow limit of the 1290 infinity II system, the flow rate was optimized for the 0.1 mm ID core–shell particle column (Kinetex XB-C18) between 0.05 and 0.1 mL/min. Operating at 0.1 mL/min provided a rapid elution within 9 min, including re-equilibration while maintaining high sensitivity, particularly for critical analytes such as E2 and E3 (Figure S5). Higher flow rates were not pursued, as the detection sensitivity significantly dropped with increasing flow. (49) Despite the rapid gradient elution, the chromatographic separation of E2 and T with their epimers (epiE2 and epiT) was achieved (Figure S3). E1 and E2 maintained baseline separation even after derivatization (Figure 1). The injection volume was optimized to 10 μL, providing maximal sensitivity without observable overloading. Minimizing extra-column volume is essential to reduce extra-column peak dispersion and prevent band broadening, which can compromise chromatographic resolution and sensitivity. (50) To address this, an external column oven was strategically installed in close proximity to the ion source inlet. Furthermore, the capillary length between the LC outlet and MS inlet was minimized to 8 cm (corresponding to a volume of 0.9 μL with a 0.12 mm i.d.) via direct connection by circumventing the default grounding unit of the ion source. A specialized grounding cable was employed to prevent additional grounding paths. Those optimizations effectively minimize extra-column volume and preserve peak integrity.
 Anal. Chem. 2025, 97, 25, 13496–13503: Figure 1. Overlay of normalized chromatograms (quantifier mass transition) of steroids in human plasma. Target analytes are shown in black, surrogate calibrants are marked in blue, and internal standards are highlighted in red. 1: cortisol-d4; 2: cortisol; 3: cortisone-d8; 4: cortisone; 5: cortisone-13C3; 6: CORT-d8; 7: CORT; 8: E3-d3 DMIS; 9: E3 DMIS; 10: dienogest-d8; 11: dienogest; 12: T; 13: T-13C3; 14: 17OHP-d8; 15: 17OHP–13C3; 16: 17OHP; 17: E2 DMIS; 18: E2-13C3 DMIS; 19: DHT-d3; 20: DHT; 21: LNG-d6; 22: LNG; 23: EE2-d4 DMIS; 24: EE2 DMIS; 25: E1-13C6 DMIS; 26: E1-13C3 DMIS; 27: E1 DMIS; 28: NoAc; 29: Preg; 30: Preg-13C2d2; 31: P-d9; 32: P–13C3; 33: P; 34: ChAc-d6; 35: ChAc; 36: ALLO-d5; 37: ALLO.
Anal. Chem. 2025, 97, 25, 13496–13503: Figure 1. Overlay of normalized chromatograms (quantifier mass transition) of steroids in human plasma. Target analytes are shown in black, surrogate calibrants are marked in blue, and internal standards are highlighted in red. 1: cortisol-d4; 2: cortisol; 3: cortisone-d8; 4: cortisone; 5: cortisone-13C3; 6: CORT-d8; 7: CORT; 8: E3-d3 DMIS; 9: E3 DMIS; 10: dienogest-d8; 11: dienogest; 12: T; 13: T-13C3; 14: 17OHP-d8; 15: 17OHP–13C3; 16: 17OHP; 17: E2 DMIS; 18: E2-13C3 DMIS; 19: DHT-d3; 20: DHT; 21: LNG-d6; 22: LNG; 23: EE2-d4 DMIS; 24: EE2 DMIS; 25: E1-13C6 DMIS; 26: E1-13C3 DMIS; 27: E1 DMIS; 28: NoAc; 29: Preg; 30: Preg-13C2d2; 31: P-d9; 32: P–13C3; 33: P; 34: ChAc-d6; 35: ChAc; 36: ALLO-d5; 37: ALLO.
Advanced sMRM was employed for data acquisition, ensuring at least two ion transitions for each target analyte, surrogate calibrant, and internal standard. Dwell weight was strategically increased for analytes anticipated at low concentrations in patient samples. Most target compounds were analyzed in positive ion mode to gain higher sensitivity with interference-free acquisition at their corresponding retention times. However, cortisol/cortisol-d4 and cortisone/cortisone-13C3 were quantified in negative ion mode due to the interference observed in positive ionization. After automatic tuning of compounds in 50% MeOH with 0.1% formic acid, the 5 most abundant mass transitions were checked for sensitivity and selectivity in the spiked matrix as a preliminary experiment, as background noise and selectivity can differ significantly between neat solution and real sample matrices. Two ion transitions per compound were selected as quantifiers and qualifiers based on their sensitivity and selectivity. Qualifier-to-quantifier ratios were monitored with an acceptance criterion of a maximum ±2 standard deviations (±2σ) compared to the average ratio in calibration runs. Ion source parameters and collision gas pressure were further optimized through systematic on-column injections to ensure robust and reliable performance.
Application
In a clinical study, the impact of hormonal fluctuations on stress levels in females using hormonal contraceptives was investigated. A total of 86 females were recruited and categorized into three groups: females using LNG-releasing intrauterine devices (IUD, n = 27), females using oral contraceptives (OC, n = 30), and females with a natural menstrual cycle (NC, n = 29). Seventy-five of the eighty-six participants completed two study visits to assess intraindividual variability. Eventually, 163 plasma samples were successfully measured with the validated LC–MS/MS methods. In this clinical measurement, in approximately one-third of the samples, concentration of ALLO and E2 were below the LLoQ (35.6 pg/mL and 3.45 pg/mL, respectively), preventing accurate quantification of these steroids. Therefore, a more sensitive method is needed specifically for ALLO and E2 quantification. Regarding E3, only around 20% of the sample had concentrations above the LLoQ (1.07 pg/mL), which was expected given that E3 is primarily present in significant levels during pregnancy.
The determined concentrations of the targets are illustrated in Figure 2 using box plots. For most endogenous hormones, including Preg, ALLO, P, 17OHP, E1, and E2, NC females exhibited the highest concentrations, followed by IUD and OC users (all p < 0.00135, Kruskal–Wallis test, applicable to the following results). Similarly, for DHT, NC females had higher concentrations than the other two groups (p = 0.00067). In contrast, the stress hormones cortisol and cortisone followed an inverse pattern, with OC users showing significantly higher concentrations compared to NC and IUD groups (p < 3.40 × 10–8). Statistical analysis of targets is summarized in Table S8. Exogenous hormones detected in the samples were LNG for IUD users and EE in combination with a progestin-most commonly LNG-for OC users. A detailed discussion and interpretation of the results is available in Bürger’s work. (53)
 Anal. Chem. 2025, 97, 25, 13496–13503: Figure 2. Boxplot distribution of endogenous and exogenous steroid concentrations in samples collected from females with a natural menstrual cycle (NC), using intrauterine devices (IUD), and using oral contraceptives (OC).
Anal. Chem. 2025, 97, 25, 13496–13503: Figure 2. Boxplot distribution of endogenous and exogenous steroid concentrations in samples collected from females with a natural menstrual cycle (NC), using intrauterine devices (IUD), and using oral contraceptives (OC).
Conclusions
This study presented a high-throughput LC–MS/MS method for steroid quantification, utilizing 96-well plate SPE for streamlined sample preparation and achieving a total analysis time of 9 min, which makes it well-suited for large-scale clinical studies. While the current vacuum evaporation step may limit applicability in time-sensitive clinical settings, it was demonstrated that nitrogen-based alternatives offer a practical solution to enhance throughput.
Sensitivity was significantly enhanced by performing low-flow (0.1 mL/min) LC separation on a narrow-diameter (1.0 mm) core–shell C18 column, optimizing ionization efficiency. Furthermore, DMIS-based selective derivatization was employed to improve estrogen detection sensitivity while preserving the integrity of other steroids. A broad steroid panel was achieved through sMRM, allowing simultaneous quantification of derivatized estrogens and nonderivatized steroids in a single analytical run. Accurate and reliable quantification was ensured through the use of 13C-labeled SIL surrogate calibrants and deuterated internal standards, effectively correcting for matrix effects and extraction variability. To our knowledge, this study is the first to report and demonstrate the feasibility of combining surrogate calibration with chemical derivatization and features a unique hybrid calibration strategy to maintain analytical validity across endogenous and exogenous target analyte classes without requiring separate analytical runs.
Method validation was performed following FDA bioanalytical method validation guidelines and supplemented with additional criteria to address the lack of formal regulatory guidance, providing a reference for future surrogate calibrant-based quantification studies. Along these lines, validation was complemented by confirming accurate quantification of certified commercial quality controls.
Further improvements in method performance are expected with increased availability of SIL standards, ideally 13C-labeled analogues, enabling even more precise quantification through optimum internal standardization.
Overall, this method integrates high sensitivity, efficient sample preparation, and a broad analytical scope, making it a powerful tool for large-scale steroid analysis in clinical and biomedical research. Future advancements in chromatography and next-generation mass spectrometers with enhanced sensitivity will further push analytical limits, enabling steroid research in challenging sample types and lower volumes (e.g., murine plasma).




