Optimization of Methodology for Simultaneous Quantification of Trigonelline, 5-Caffeoylquinic Acid, and Caffeine in Green and Roasted Coffee Extracts by HPLC
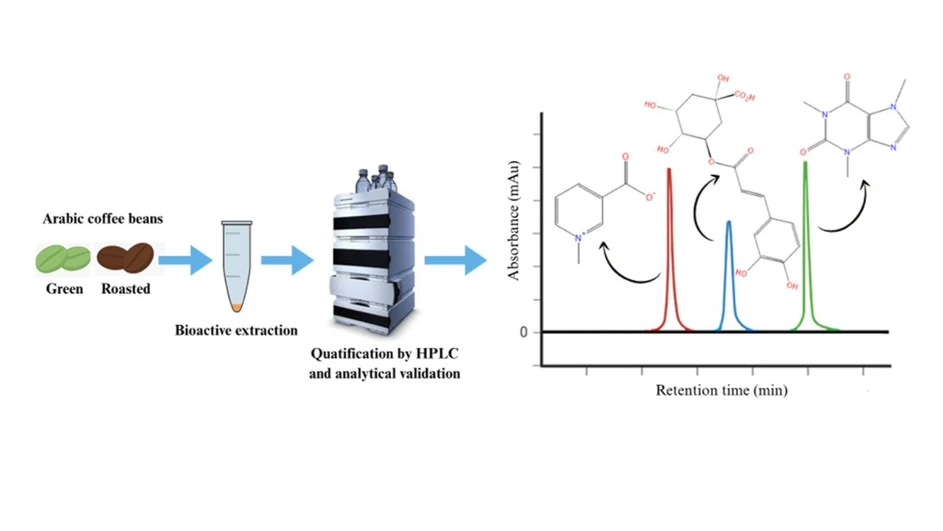
ACS Omega 2025, 10, 35, 40304–40312: Graphical abstract
Coffee quality is strongly influenced by key bioactive compounds such as trigonelline, 5-caffeoylquinic acid (5-CQA), and caffeine. This study optimized and validated a fast, accessible HPLC-UV method for their simultaneous quantification in five Coffea arabica cultivars. The method demonstrated excellent linearity, accuracy, precision, robustness, and suitable detection limits, meeting analytical validation criteria.
Application of the method revealed notable differences among cultivars, including the lowest caffeine level in Laurina and variable degradation of trigonelline and 5-CQA after roasting. The Arara cultivar showed the greatest 5-CQA loss, while Laurina exhibited the highest trigonelline degradation. Due to its speed and simplicity, the method is well suited for investigating how these compounds influence sensory attributes and potential health benefits.
The original article
Optimization of Methodology for Simultaneous Quantification of Trigonelline, 5-Caffeoylquinic Acid, and Caffeine in Green and Roasted Coffee Extracts by HPLC
Walace Breno da Silva*, Larissa Martins Rocha, Lucca Dornelas Guimarães Moura, Márcio Santos Soares, Sabrina Alves da Silva, Daniele Birck Moreira, Pedro Ivo Vieira Good God, Geraldo Humberto Silva
ACS Omega 2025, 10, 35, 40304–40312
https://doi.org/10.1021/acsomega.5c05526
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Various analytical techniques have been employed in the characterization of bioactive compounds and in the authentication of coffee, including ultraviolet (UV) spectroscopy and liquid chromatography coupled with mass spectrometry (LC-MS). (16) Recent studies have demonstrated the feasibility of quantifying compounds such as sucrose, caffeine, and trigonelline in green coffee beans using advanced techniques such as hyperspectral imaging (HSI), although these approaches require sophisticated instrumentation and involve higher analytical complexity. (17) Simplified methodologies have been proposed for the determination of alkaloids and phenolic acids in coffee, such as the use of the QuEChERS method coupled with ultraviolet–visible (UV–vis) spectrophotometry for detecting trigonelline, caffeine, and 5-caffeoylquinic acid in green coffee bean extracts. (18) However, this approach presents limitations, as the spectral signals of caffeine and chlorogenic acids overlap in the 200–500 nm range, hindering their simultaneous quantification in simple aqueous extracts. (19) Therefore, UV–vis spectroscopy lacks adequate specificity in complex matrices, such as coffee infusions.
From a chromatographic standpoint, HPLC-based methodologies have proven to be effective for the simultaneous quantification of these compounds. For instance, Santiago et al. (2020) developed a method for the simultaneous analysis of trigonelline, caffeine, and 5-caffeoylquinic acid with a total chromatographic runtime of 20 min. (20) Additionally, Mehari et al. (2015) reported the application of HPLC in the quantification of alkaloids in green coffee beans, including trigonelline, caffeine, theobromine, and theophylline. However, due to the naturally low concentrations of theobromine and theophylline in coffee beans, the methodology has limitations in simultaneously quantifying all target components in the coffee matrix. (21)
Nevertheless, these methods often require elaborate sample preparation steps, such as extractions with organic solvents and filtration, which negatively impact their applicability in routine analyses and their sustainability profile. In this context, this study proposes a more straightforward and sustainable approach for the simultaneous analysis of trigonelline, 5-caffeoylquinic acid, and caffeine in extracts from both raw and roasted coffee beans. This method is based solely on the direct infusion of ground coffee beans with hot water, eliminating the use of organic solvents and reducing the sample preparation time. The analysis is performed using high-performance liquid chromatography with a diode array UV detector (HPLC-UV-DAD), with a short analysis time. Accurate determination of these compounds contributes to a better understanding of the chemical and sensory characteristics of coffee, facilitating the standardization and optimization of postharvest processes to enhance and ensure product quality, thereby adding value. Furthermore, proper method validation increases the reliability of results, enabling comparisons across different studies and laboratories. (22)
2. Materials and Methods
2.2. Equipment
An Agilent 1260 HPLC liquid chromatograph equipped with a G7111B 1260 Quat pump, a G7129A 1260 Vial sampler autosampler, and a G7117C 1260 DAD HS detector were used (Agilent, Santa Clara). The column used was a Luna Omega Polar C18 LC column with an internal diameter of 4.6 mm, length of 150 mm, and particle size of 5 μm (Phenomenex, Aschaffenburg, Germany) connected to an Eclipse XDB-C18 precolumn with an internal diameter of 4.6 mm, length 12.5 mm, particle size 5 μm (Agilent, Santa Clara).
3. Results and Discussion
3.1. Method Validation
To check the quality and resolution of the chromatograms of the raw and roasted coffee extracts, the standard was added to the raw and roasted extracts, and the same concentration was added to both extracts (10 μg mL–1) in triplicate. An elution time of less than 5 min was observed for the analytes and good resolution of the chromatographic bands (Figure 2).
 ACS Omega 2025, 10, 35, 40304–40312: Figure 2. Chromatogram with the analytes trigonelline (band a), 5-CQA (band b), and caffeine (band c). Conditions: Luna Omega Polar C18 column (internal diameter 4.6 mm, length 150 mm, particle size 5 μm); mobile phase: water with 1% acetic acid: acetonitrile (85:15); flow rate 1 mL min–1; detection: UV 272 nm, roasted coffee (1) and raw coffee (2), k’ is the retention factor; N is the number of theoretical plates; S is the symmetry; Rs is the resolution, and Se is the selectivity.
ACS Omega 2025, 10, 35, 40304–40312: Figure 2. Chromatogram with the analytes trigonelline (band a), 5-CQA (band b), and caffeine (band c). Conditions: Luna Omega Polar C18 column (internal diameter 4.6 mm, length 150 mm, particle size 5 μm); mobile phase: water with 1% acetic acid: acetonitrile (85:15); flow rate 1 mL min–1; detection: UV 272 nm, roasted coffee (1) and raw coffee (2), k’ is the retention factor; N is the number of theoretical plates; S is the symmetry; Rs is the resolution, and Se is the selectivity.
The results for the theoretical plates were above the acceptance criteria of the Food and Drug Administration, (28) which states that they should be higher than 2000. The symmetry of the bands was above 0.65, which indicates that the characteristics of the compounds influence the symmetry. Better symmetry can be obtained by changing the chromatographic conditions, such as column pressure and mobile phase flow rate. (29) Despite the symmetry values being higher than 0.65, the evaluated accuracy and precision demonstrate that the method is reliable. Trigonelline had a k’ of less than 1, which shows its low affinity with the stationary phase. Resolution and selectivity were also affected by trigonelline’s poor interaction with the stationary phase, which was not the case with 5-CQA and caffeine.
3.2. Application of the Method to Quantify Bioactive Compounds
Validation of the method showed that the results were within established standards, confirming its suitability for quantification of bioactive compounds in coffee. This quantification helps us to study and understand how these compounds influence the sensory characteristics of the beverage. The method was then applied to measure three specific compounds in five varieties of Coffea arabica, each processed differently after harvest.
The concentration of trigonelline in raw coffee beans varied between 13.38–15.24 mg g–1 (Table 8), while in roasted beans, it was between 10.68–12.75 mg g–1, values similar to those found by Mehari. (21) Trigonelline varies between 10 and 22 mg g–1 in raw beans and is generally 10 mg g–1 or less in roasted beans. (32) The Tukey test revealed significant variation in trigonelline content among the roasted coffee samples, with Yellow Catuai and Guesha exhibiting the highest concentrations. In the raw beans, Yellow Catuai and Arara stood out with lower trigonelline levels compared with the other cultivars.
Caffeine concentrations ranged from 7.93 to 12.72 mg g–1 in raw and roasted coffee beans, similar to those found in the literature. (21) When comparing raw and roasted beans of the same variety, it was observed that there was a significant variation in concentration only in Guesha beans. The lack of significant variation between the other four varieties is because caffeine belongs to a class of compounds called methylxanthines, which is considered a natural ergogenic that is not degraded or hydrolyzed during the roasting process. (41) Degradation was significant only for the Guesha variety (15%) and may be related to the roasting and processing processes to which the beans were subjected. The method proved to be efficient in showing that the Laurina genotype has a lower caffeine content, as reported in the literature.
Recent studies, such as those by Santanatoglia et al. and Farag et al., confirm the reliability of LC-MS/MS for the simultaneous determination of caffeine, trigonelline, and chlorogenic acids, serving as a comparative basis for validating more accessible methods. (42,43) The good agreement between the values obtained by the proposed methodology and the LC-MS/MS data reinforces its applicability as a viable alternative for quality control and phytochemical analysis laboratories, especially in contexts where access to highly complex techniques is limited. The proposed methodology can be compared with established reference methods, such as ISO 20481:2008 and AOAC 979.08, which establish chromatographic parameters for the quantification of caffeine by HPLC-UV in coffee matrices. (44,45) These methods utilize a mobile phase with a higher proportion of organic solvents and more elaborate sample preparation steps (such as reflux extraction). The methodology proposed in this work offers shorter analysis time and lower environmental impact, representing a cleaner, safer, and faster analytical alternative suitable for industrial quality control and laboratory studies.
In conclusion, the method developed for the simultaneous analysis of trigonelline, 5-caffeoylquinic acid, and caffeine in extracts of raw and roasted coffee using HPLC-UV has demonstrated effectiveness in terms of linearity, accuracy, and precision for the concentrations tested. This method offers a rapid and efficient alternative for analyzing the compositions of these bioactive compounds in coffee samples. The concentrations of trigonelline, 5-caffeoylquinic acid, and caffeine vary significantly among different coffee samples. However, the most important variation is observed in 5-caffeoylquinic acid. This compound may be essential for understanding how the composition of chlorogenic acids affects the characteristics of different coffees.