Quality-by-Design-Driven RP-HPLC Method Development and Validation for Impurity Analysis of Elexacaftor, a Cystic Fibrosis Drug, with LC-MS/MS-Based Degradant Identification
ACS Omega 2025, 10, 39, 44885–44894: Graphical abstract
A stability-indicating RP-HPLC method was developed and validated for impurity analysis of the cystic fibrosis drug Elexacaftor using a Quality-by-Design (QbD) approach. The method achieved effective separation of six known impurities with appropriate resolution and peak shape under optimized chromatographic conditions.
Validation according to ICH guidelines confirmed the method’s robustness, linearity, accuracy, specificity, and precision. Stress testing under hydrolytic, oxidative, thermal, and photolytic conditions demonstrated suitability for degradation studies, supporting both process development and routine quality control of bulk drug substances.
The original article
Quality-by-Design-Driven RP-HPLC Method Development and Validation for Impurity Analysis of Elexacaftor, a Cystic Fibrosis Drug, with LC-MS/MS-Based Degradant Identification
Jayaprakash Kanijam Raghupathi, Divya Kumar Vemuri, Syed Mastan Ali, Vishnuvardhana Kishore Polisetti, Rambabu Gundla, Leela Prasad Kowtharapu, Dittakavi Ramachandran*, and Naresh Kumar Katari*
ACS Omega 2025, 10, 39, 44885–44894
https://doi.org/10.1021/acsomega.4c10834
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Elexacaftor is a drug that is used to treat fibrosis (TCF). When treating patients with cystic fibrosis (CF) who have the F508del mutation or other genetic mutations that react well with these medications, it is specifically used in conjunction with two other medications, ivacaftor and tezacaftor. The cystic fibrosis transmembrane conductance regulator, or CFTR, modulator, is Elexacaftor. Due to genetic abnormalities, people with CF have abnormal CFTR protein function, which is improved by this treatment. Based on clinical research, individuals with CF should expect a much better quality of life, reduced pulmonary exacerbations, and improved lung function when using Elexacaftor, ivacaftor, and tezacaftor together as opposed to prior treatments. After receiving FDA approval, Elexacaftor was marketed in the USA under the brand name Trikafta in October 2019. (1−5) Since then, it has emerged as a key component of the CF treatment plan, especially for those with specific mutations. It is taken orally as tablets or granules, usually once a day, along with a meal high in fat. Headache, upper respiratory tract infection, abdominal pain, diarrhea, rash, and elevated liver enzymes are among the frequently reported side effects. Generally speaking, these side effects are controllable and can be well watched during therapy.
To ensure the quality and safety of ELX, it is required to develop analytical methods for the identification and estimation of impurities. The currently available literature reveals that the ELX drug combination with ivacaftor and tezacaftor is used very effectively. So, most of the analytical methods related to the estimation of three drugs simultaneously quantitatively by using different analytical techniques, i.e., by high-performance liquid chromatography and liquid chromatography–mass spectroscopy. Several liquid chromatography-ultraviolet (LC-UV) methods (6,7) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) (8−15) methods are available to simultaneously measure the concentration of different combinations of IVA/TEZ/ELX with metabolites in plasma or other biological fluids. However, we did not find the literature for the analysis of process-related impurities and degradation impurities. The methods available in the literature do not discuss the process-related impurities and the degradation impurities of the ELX drug substance; hence, a stability-indicating LC method was developed for identifying and estimating the process-related impurities and degradation impurities of the ELX drug substance. In this paper, we report on a new analytical method to the estimation of ELX and its six process-related impurities quantitatively by using an HPLC instrument. This new LC method is sensitive enough to detect impurities as low as 0.02%, which ensures there is no harm or risk to the patients from impurities; however, existing methods in the literature do not discuss the sensitivity of the potential impurities, which can harm the patients.
The method has been validated according to current international guidelines; this method is specific and rugged for analysis. An experimental design constructed according to Quality by Design (QbD) can identify the individual effects as well as interactions of the critical method variables, such as organic ratio, pH of the buffer or mobile phase, flow rate, column oven temperature, and gradient composition. (16−18) It is a systematic approach to analytical method development that is risk-based, which helps to create robust and reliable analytical methods. The current robustness method was studied by utilizing the QbD-based Design of Experiments (DoE) with Design-Expert software.
2. Materials and Methods
2.2. Instrumentation and Software
Waters Alliance e2695 HPLC with a quaternary pump was used for method development and analysis (34 Maple St, Milford, Massachusetts 01757, USA). Empower 3 software (Waters Corporations, USA) was used for data collection and processing. Mettler Toledo microbalance was used, on which weighing was done on an MX5 microbalance (Mettler Toledo, Columbus, Ohio, USA). A SevenMulti pH meter (Mettler Toledo Columbus, Ohio, USA) was used for estimation of the pH.
2.3. HPLC Instrumentation and Conditions
Waters Alliance’s e2695 LC system with the 2998 photodiode array (PDA) detector was used for method development, method validation, and forced degradation investigations. Using Empower software, data were gathered and processed. Using a Waters 2998 PDA detector, the peak homogeneity was investigated.
2.3.1. Chromatographic Method Conditions
The analysis of Elexacaftor employed the XBridge Shield RP18, 150 mm × 4.6 mm, 3.5 μm column with 10 mm KH2PO4 in water, pH adjusted to 3.2 with phosphoric acid buffer, and showed positive and encouraging results. Furthermore, extensive trials were made on an XBridge shield RP column with various flow rate and gradient conditions. The optimum separation of all impurities and ELX was achieved on XBridge Shield RP18, 150 × 4.6 mm, 3.5 μm, with mobile phase A as buffer:methanol (98:2, v/v) and mobile phase B as acetonitrile:2-propanol:water (70:20:10, v/v/v), flow rate 0.7 mL/min; column temperature 27 °C; with binary gradient time (min)/B concentration (%): 0/5, 20/50, 60/85, 80/85, 82/5 and 90/5. All peaks were separated with good resolution and peak shape under these conditions and are shown in Figure 2. The diluent was a 40:60 v/v solution of water and acetonitrile that was also used to wash the needles.
3. Results and Discussion
3.2. Optimized Chromatographic Conditions
Based on the method developmental trials, it was found that Elexacaftor and related substances were not retained and separated as per the requirement in any of the screened conditions except the trial with XBridge Shield RP 18, 150 mm × 4.6 mm, 3.5 μm, mobile phase A as buffer:methanol (98:2, v/v) and mobile phase B as acetonitrile:2-propanol:water (70:20:10, v/v/v), flow rate 0.7 mL/min; column temperature 27 °C; with binary gradient time (min)/B conc (%): 0/5, 20/50, 60/85, 80/85, 82/5, and 90/5. All seven peaks were separated with good resolution and peak shape in this condition. The details of the finalized method conditions are mentioned in Table 2 for quantitating ELX and its related substances by using HPLC.
 ACS Omega 2025, 10, 39, 44885–44894: Table 2. Analysis of Optimized HPLC Method Conditions for Elexacaftor and Its Impurities
ACS Omega 2025, 10, 39, 44885–44894: Table 2. Analysis of Optimized HPLC Method Conditions for Elexacaftor and Its Impurities
With the above chromatographic conditions, ELX and its related substances (Imp-A to Imp-F) got separated from each other with a resolution of more than 2.0, as shown in Figure 3.
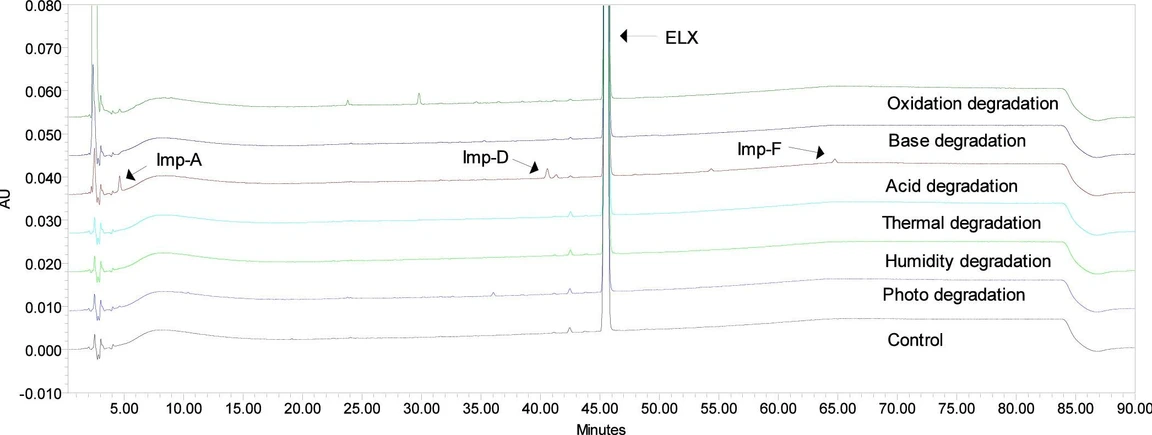 ACS Omega 2025, 10, 39, 44885–44894: Figure 3. Overlay chromatogram of degradation studies.
ACS Omega 2025, 10, 39, 44885–44894: Figure 3. Overlay chromatogram of degradation studies.
3.3. Method Validation Results
According to ICH requirements, (19−23) the created approach was validated, and the results are shown in Table 3. In the presence of its process contaminants, the devised HPLC technique for ELX’s specificity was assessed. ELX and Imp-A-Imp-F are well separated from one another. Utilizing a photodiode array detector and HPLC, the analysis was completed. All of the peaks of ELX and its impurities were shown to be pure when the peak purity tool was employed on them, indicating the method’s specificity because no additional impurities were detected coeluting at the same retention period.
 ACS Omega 2025, 10, 39, 44885–44894: Table 3. Method Validation Summary of the Estimation of Elexacaftor and Its Impurities_Part 1
ACS Omega 2025, 10, 39, 44885–44894: Table 3. Method Validation Summary of the Estimation of Elexacaftor and Its Impurities_Part 1
 ACS Omega 2025, 10, 39, 44885–44894: Table 3. Method Validation Summary of the Estimation of Elexacaftor and Its Impurities_Part 2
ACS Omega 2025, 10, 39, 44885–44894: Table 3. Method Validation Summary of the Estimation of Elexacaftor and Its Impurities_Part 2
4. Conclusions
For the measurement of Elexacaftor and its related substances (imp-A to imp-F and any other impurities), the suggested technique was optimized to give a sharp peak with a minimal tailing factor. This approach was found to be specific, accurate, linear, and economical. The approach offers a number of benefits, including cost-effectiveness, robustness, and increased sensitivity. It was the first time we developed a method to estimate the content of Elexacaftor drug alone in process development. The current technique has been demonstrated to be efficient in terms of cost and may be utilized to measure Elexacaftor and its related compounds in the bulk. ICH rules were followed in the method’s validation.




