Menu
More information
WebinarsAbout usContact usTerms of use
LabRulez s.r.o. All rights reserved. Content available under a CC BY-SA 4.0 Attribution-ShareAlike
Scientific article | Science and research
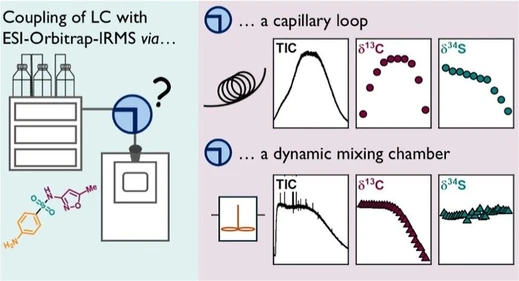
Coupling Liquid Chromatography to Orbitrap Isotope Ratio Mass Spectrometry: Overcoming Isotope Effects of Chromatography and Amount-Dependency by Peak Homogenization
This study couples LC to ESI-Orbitrap MS for stable isotope analysis using peak homogenization to remove chromatographic isotope effects and amount dependency, achieving precise C, N, and S isotopologue ratios.
Mo, 26.1.2026
LabRulez


Article | Science and research
MOSH/MOAH Analysis: Technology, Regulation, and the Road Ahead
MOSH/MOAH testing is becoming a key topic in food safety and regulation. Discover how Thermo Fisher Scientific’s LC-GC-FID and GC×GC-MS workflows improve reliability, compliance, and source identification.
Tu, 28.10.2025
Thermo Fisher Scientific

More information
WebinarsAbout usContact usTerms of use
LabRulez s.r.o. All rights reserved. Content available under a CC BY-SA 4.0 Attribution-ShareAlike