
Waters BioAccord LC-MS System
Routine biopharmaceutical analysis – accessible and accelerated
Improving lab efficiency without sacrificing result quality is important but challenging. Now you can achieve high-quality biotherapeutic molecule data while maximizing efficiency with the ability to directly measure product attributes to assess product quality and process robustness.
The BioAccord LC-MS System is specifically designed for routine definition and monitoring of biotherapeutic attributes across biopharmaceutical process and product development, manufacturing, and QC, all within a small system footprint on a platform capable of operation by non-MS experts.
Analyze a broad range of product attributes using the intact molecule, subunits, peptide maps, and released glycans, all with greater sensitivity, selectivity, and dynamic range than traditional chromatographic and electrophoretic techniques.
- Make rapid, confident decisions with accessible, consistent high-quality data
- Ensure operational simplicity, result consistency, and lab productivity with workflow automation
- Compliant-ready platform that can be deployed and replicated from discovery through to QC.
- Achieve fast deployment, reliable operation, and a clear return on investment
Overview
- Compliant-ready platform ideal to deploy from discovery to quality control (QC)
- Achieve faster time to value, and consistent performance through automated set-up with SmartMS
- Diagnose, and fix potential system issues before they affect data quality with intelligent health checks in SmartMS
- Focus your team on what matters with a system that doesn't require LC-MS experts for routine operation
- Make more informed, data-driven decisions with trustworthy LC-MS data directly available at the point of decision-making
- Achieve the highest quality data in your lab, comparable to data previously available only from the complicated, and costly LC-MS systems used for product characterization
Recommended Use: For the rapid measurement of multiple product quality attributes during routine biopharmaceutical analysis.
 Waters BioAccord LC-MS System
Waters BioAccord LC-MS System
Accelerate decisions with less effort to collect and process high quality data
Minimize the time spent generating data and managing LC-MS performance and maximize time for actions driven by the high quality data generated with automated set-up and guided workflows.
Use the streamlined workflows with the BioAccord LC-MS System for:
- Intact mass analysis (proteins, subunits, oligonucleotides, mRNA fragments)
- Peptide mapping and multi-attribute method (MAM)
- Released N-glycan analysis
- Oligonucleotide sequence confirmation
- Impurity analysis for proteins, peptides, and oligonucleotides
These workflows streamline method set-up, data acquisition and processing, and report generation to deliver the required information with minimal manual interventions. In addition to these core workflows, the BioAccord System also supports a range of other applications required in biotherapeutic process and product development, like cell culture media analysis, mRNA cap, and lipid nanoparticle analysis.
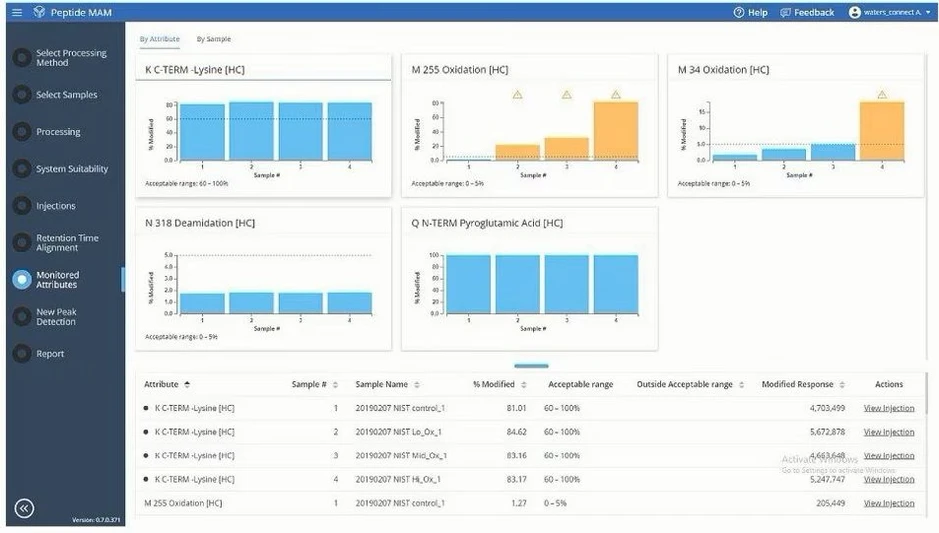 Waters BioAccord LC-MS System: Visualize your data with intuitive and easy to review layouts. These results from a peptide MAM analysis show product quality attributes that are being monitored, flagging those that are out of specification.
Waters BioAccord LC-MS System: Visualize your data with intuitive and easy to review layouts. These results from a peptide MAM analysis show product quality attributes that are being monitored, flagging those that are out of specification.
Increase productivity by increasing data quality and reducing analysis failures
Non-specific adsorption of metal sensitive analytes can be unpredictable and challenging, leading to long system passivation times, large relative standard deviations (RSDs), and broad peaks that can be tough to detect and quantify.
The BioAccord LC-MS System incorporates ACQUITY Premier UPLC and Premier chemistries with MaxPeak High Performance Surfaces (HPS) Technology. These innovations maximize system readiness, decrease time to operation, and enable superior run-run and system-system consistency while improving sensitivity for the metal interacting analytes.
Through the application of MaxPeak HPS Technology, you can:
- Increase recovery and consistent detection of problematic peptides for enhanced peptide map and MAM analysis.
- Achieve superior sensitivity and reproducibility for acidic glycans and lipids by minimizing analyte/surface interactions that lead to sample loss.
- Save time and improve productivity by reducing or eliminating passivation for robust oligonucleotide analyses.
- Minimize the risk of undetected peaks by minimizing metal-sensitive analyte loss, improving recovery and the lowering limits of detection.
 Waters BioAccord LC-MS System: The figure (A) shows the extracted ion chromatograms (XIC) for GFYPSDIAVEWESNGQPENNYK (PENNYK) peptide unmodified and deamidated peptides collected for a NISTmAb digest. The chromatographic trace in solid line shows the XIC for PENNY peptides acquired on the BioAccord System. The inset shows all modification of PENNY peptide including succinimide modification. Figure (B) shows the total normalized peak area calculated for PENNY peptides using conventional and ACQUITY Premier UPLC system.
Waters BioAccord LC-MS System: The figure (A) shows the extracted ion chromatograms (XIC) for GFYPSDIAVEWESNGQPENNYK (PENNYK) peptide unmodified and deamidated peptides collected for a NISTmAb digest. The chromatographic trace in solid line shows the XIC for PENNY peptides acquired on the BioAccord System. The inset shows all modification of PENNY peptide including succinimide modification. Figure (B) shows the total normalized peak area calculated for PENNY peptides using conventional and ACQUITY Premier UPLC system.
Obtain quality data, faster than ever before
Quickly getting the quality data you need for your laboratory to stay productive is paramount but deploying traditional LC-MS systems is often rather slow. The integrated nature of the BioAccord LC-MS System means the system can be deployed easily and users can be productive faster through the delivery of system and applications training with appropriate biopharmaceutical methods, sample data, and system check standards for the common biopharmaceutical analysis workflows.
 Waters BioAccord LC-MS System: Mass accuracy summary for 48 injections that representing six antibodies with eight replicate injections each. Mass accuracy summary for 48 injections that representing six antibodies with eight replicate injections each.
Waters BioAccord LC-MS System: Mass accuracy summary for 48 injections that representing six antibodies with eight replicate injections each. Mass accuracy summary for 48 injections that representing six antibodies with eight replicate injections each.
With the BioAccord LC-MS System, you can:
- Ensure optimal system performance with biomolecule-based installation testing.
- Enable users to quickly get up to speed with outcome-based application training.
- Achieve high quality data with existing personnel or respond quickly to staff and project turnover challenges with reduced training burdens.
Meeting your regulatory requirements, together
Meeting corporate and regulatory expectations for compliance and data integrity is critical to your laboratory operations, and those needs don’t change because LC-MS technology is moving into roles within manufacturing and quality control (QC) organizations.
Your data quality and compliance are our priority, too. The BioAccord System is built on the compliant-ready waters_connect informatics platform and backed by the long history at Waters of delivering compliant informatics and professional qualification services to streamline deployment.
With Waters, you’ll get:
- A single informatics platform based on a secure relational database architecture
- Full audit trail for acquisition, processing, and reporting of data
- Administrator-configurable roles and permissions
- Network deployment of systems for maximum data safety and controlled accessibility
- Characterization (Q-Tof) and routine analysis (BioAccord) systems on a common network for efficient system, data, and information management
- Professional qualification services

News
