Quantitative Assessment of a Novel Device Designed for Patient-centric Sampling of Dried Plasma Using Targeted Proteomics
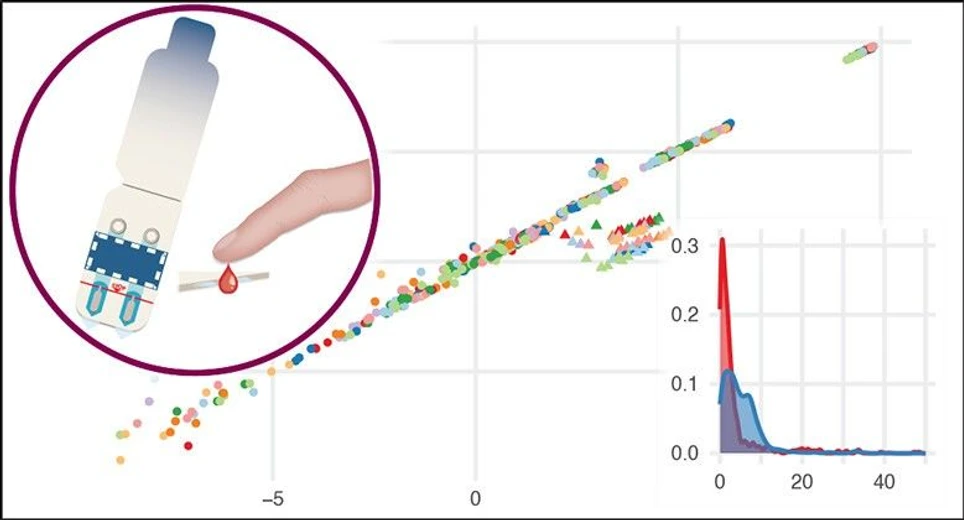
Anal. Chem. 2025, 97, 25, 12953–12962: Graphical abstract
This study evaluates a novel patient-centric microsampling device that produces dried plasma from finger-prick blood for targeted proteomic analysis. Using stable isotope-labeled standards and selected reaction monitoring, the device demonstrated high quantitative performance across a wide concentration range of plasma proteins.
The dried plasma samples showed excellent correlation with conventional plasma (R > 0.99) and strong precision, with CVs under 10% for 80% of quantified peptides. The samples also retained stability at room temperature for up to 232 days, supporting the device's utility for home-based, longitudinal sampling in large-scale proteomic studies.
The original article
Quantitative Assessment of a Novel Device Designed for Patient-centric Sampling of Dried Plasma Using Targeted Proteomics
Andreas Hober*, Marcus Henricsson, Tim Ruckh, Pia Davidsson, Benjamin Challis, Tasso Miliotis*
Anal. Chem. 2025, 97, 25, 12953–12962
https://doi.org/10.1021/acs.analchem.4c05455
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
The consequences of the healthcare restrictions due to the coronavirus disease 2019 (COVID-19) pandemic had severe implications on healthcare services that were partially or entirely disrupted in many countries. It also impacted clinical trials, particularly site opening, trial activation, and participant recruitment. (1,2) The pharmaceutical industry has been working on the decentralization of clinical trials long before the COVID-19 pandemic. (3−5) During the past decade, home sampling utilizing dried sample matrix spot approaches has gained significant traction by pharmaceutical companies, where various dried blood spots (DBS), as well as dried urine methods, have been implemented in clinical trials. (6−9) Nevertheless, the recent global pandemic has had a catalytic effect on allocating more resources regarding patient-centric microsampling. The driving forces for using home sampling in clinical studies have been to use technology that retains more patients as active participants in clinical trials.
The utilization of finger pricking and collection of 30–60 μL of capillary blood instead of drawing several milliliters of venous blood when visiting a phlebotomist in a point-of-care facility will decrease patient burden. In fact, the necessity for centrifugation of blood samples and the requirement of cold-chain storage handling remain a substantial obstacle in bringing the sampling event closer to the patient to enable self-sampling or sampling by nonprofessionals. Moreover, dry ice transportation is typically required for conventional liquid samples (e.g., blood, plasma, urine), and international shipment is subjected to strict regulations that add significantly to costs and timelines. (10,11) In contrast, the shipment of dried samples is drastically less complex, as simple envelopes can be sent by standard mail or courier services. It has also been shown that proteins stored as dried blood or plasma on paper are stable for at least 14 days when stored at room temperature (12,13) without the need for refrigeration or cold chain storage. Björkesten et al. established that the drying process was reproducible and barely influenced the detection of blood proteins. (14) They concluded that the detection of some proteins was not significantly affected even following storage for 30 years if the dried blood samples had been stored at either +4 or −24 °C.
For the longitudinal monitoring of a larger population to be successful, sample collection must be cost-efficient and convenient for the patient while producing reliable results. Microsampling can meet these requirements by circumventing traditional venipuncture sampling and cold-chain transportation. This approach also promotes carbon emission reduction and increases trial accessibility, which can facilitate participation by previously underserved populations. (15)
Microsampling using DBS techniques has several limitations, such as variable spot sizes and spot homogeneity, resulting in inconsistent concentration results due to variability in hematocrit levels. (16−18) Another disadvantage of DBS sampling, from an analytical perspective, is that the sample is contaminated with variable amounts of intracellular components originating from leukocytes and erythrocytes. The presence of these components may cause matrix interferences as well as ionization suppression in mass-spectrometry-based assays, which may result in lower detection sensitivity. These complexities lead to measurement variability from DBS samples, but they may be mitigated by generating dried plasma spots (DPS). Today, blood plasma is a well-established matrix in laboratory medicine. Thus, DPS is a promising microsampling technique, and the dried plasma matrix is expected to lead to the same quantitative result that neat plasma would for an analytical measurement.
The advantages of collecting, drying and transporting small, dried samples have supported quantitative assay development for therapeutic drug monitoring. (19−23) The first-generation DPS cards utilized either vertical (22) or lateral (24,25) flow separation membranes (e.g., Whatman CF12, Ahlstrom 226, or Munktell TFN) to produce cell-free plasma. However, both of these formats are associated with challenges, including variable sample output related to hematocrit values. (26−28) It has also been shown that lateral flow devices have unrestricted plasma flow across an undefined collection area, resulting in a concentration gradient of analytes due to chromatographic effects. (25) These quantitative sampling shortcomings have been addressed by commercial manufacturers and academic groups by implementing volumetric control of the sampling, circumventing these issues to enable precise and reproducible sampling. (29−33)
In this study, we evaluated a novel DPS microsampling device, Capitainer Plasma, from Capitainer AB (Stockholm, Sweden), a prototype of a product that will be launched at the end of 2024 with the name Capitainer SEP10. The device prepares a volume-defined dried plasma sample from a fingerprick capillary blood sample with a reported volume precision below 3% (coefficient of variance (CV)). (34) Briefly, the DPS card is based on passive filtration and utilizes an integrated metering channel for sampling a precise and consistent plasma volume, which is stored in a precut paper disk. We assessed the device quantitatively through a targeted proteomics approach. We used a selection of stable isotope-labeled protein fragments as standards for plasma proteins ranging from 1300 μM to 30 nM. Each standard corresponds to one single target protein fragment and releases its peptides upon digestion, accounting for potential digestion kinetic effects. This allows for a more robust quantification that enables the use of multiple peptides per protein target. (35,36) All samples were analyzed using selected reaction monitoring, and to ensure quantitative robustness, the device was assessed both from a storage perspective and across a group of healthy donors to ensure reliable performance between individuals.
Methods
Method Development for MS Analysis
Stable isotope-labeled recombinant protein fragments (qRePS, ProteomEdge, Stockholm, Sweden) were reduced, alkylated, and digested with trypsin (SoLu-trypsin, Sigma-Aldrich, Saint Louis, MO, USA). Briefly, 50 pmol of qRePS was diluted in 1× PBS (Gibco) and 10 mM TCEP (Bond-Breaker TCEP Solution, Thermo Fisher Scientific) to a total volume of 40 μL and incubated in a ThermoMixer (Eppendorf, Hamburg, Germany) at 60 °C and 850 rpm for 30 min. All standards were subsequently alkylated by the addition of 10 μL of 200 mM 2-chloroacetamide (CAA) to a final concentration of 40 mM and incubated at room temperature (RT) for 30 min. One microgram of trypsin was added to each standard, and the standards were digested overnight in a ThermoMixer at 37 °C and 850 rpm. The digestions were quenched by the addition of 10 μL of 10% formic acid (FA). Approximately 6 pmol of each digested standard (Table S1) was individually loaded onto the LC system. All targets were screened for any +1 and +2 product ions (b- and y-ions) from their corresponding proteotypic +2 and +3 precursor ions (5–25 amino acids). A maximum of 10 transitions were kept per precursor ion and subjected to collision energy optimization. The final transition list and the corresponding collision energies can be seen in Table S2. All method development was performed using a Thermo TSQ Altis instrument (Thermo Fisher Scientific, Waltham, MA, USA) connected to a Vanquish Horizon LC system (Thermo Thermo Fisher Scientific). The LC system was equipped with a 150 mm Acclaim VANQUISH C18 column (P/N 071399-V, Thermo Fisher Scientific) and operated with an LC gradient as specified in Table S3.
LC–MS/MS Analysis of Samples for Quantitative Benchmarking and Long-Term Stability
All samples from the nine donors and stability experiment were analyzed by loading approximately 10 μg of peptides onto a Thermo Vanquish NEO (Thermo Fisher Scientific) LC system coupled to a Thermo TSQ Altis (Thermo Fisher Scientific). The LC system was operated in microflow mode and equipped with a 5 mm trapping column (YMC-Triart C18, P/N TA12S03-E5H0 AU, YMC CO., Ltd., Kyoto, Japan), a 150 mm analytical column (YMC-Triart C18, P/N TA12SP9-15H0 AU, YMC CO., Ltd.) and a stainless-steel spray needle (PepSep emitter, P/N PSFSELJ20, Bruker Daltonics, Bremen, Germany). The mobile phase of the LC system consisted of solvent A (0.1% FA) and solvent B (acetonitrile and 0.1% FA). The samples were analyzed using the LC gradient specified in Table S3.
Results
Quantitative Benchmarking
To assess the reproducibility of the correlation observed in the elution optimization, paired plasma and microsampling samples were collected from nine healthy donors in duplicates. The samples were eluted using the protocol established in the previous experiments, and either 40 μL of eluate or 1 μL of neat plasma was spiked with internal standards. The samples were digested and analyzed by LC–MS/MS. To ensure that the quantitative comparison would not be directly impacted by data with low signal-to-noise ratio, all included peptides had to be quantified in all samples. This resulted in the exclusion of six protein targets. All microsampling samples were normalized to albumin.
As seen in the optimization of the elution protocol, the correlation between the microsampling samples and the plasma is observed across all healthy donors sampled and remains consistent (Figure 3A) with an R2 of 0.99. We still observe an outlier cluster consisting of the peptides from FGB. The precision of the measurements remains stable, and the majority of peptides, in both the plasma samples and microsampling samples, have a CV below 10% (Figure 3B). All protein targets quantified have been visualized as boxplots for both neat plasma and the microsampling device and are presented in Figure S2. The median CV values were 1.6% and 4.2% for microsampled samples and neat plasma, respectively. Figure 3C and Figure 3D illustrate the molar difference observed between the plasma samples and the microsampling samples at a peptide and protein level in percent after normalization, using the absolute quantification in neat plasma as the reference value. It is noteworthy that the peptides with the most discernible difference observed are the peptides from the FGB cluster and the peptides with the lowest molar amount. However, the majority of the targets exhibit minimal difference between the two sampling strategies, with a median difference of −1.8% at a peptide level. Seventeen of the 20 quantifiable targets exhibited a difference below 20%, compared to neat plasma at a protein level, with a median difference of −1.6%.
 Anal. Chem. 2025, 97, 25, 12953–12962: Figure 3. (A) A scatterplot of all peptides quantified in the nine healthy donors, showing a strong correlation. The peptides are plotted with the normalized log2(ratio to standard) from the microsampling device on the y-axis and the normalized log2(ratio to standard) from the plasma on the x-axis. (B) The precision of the measurements in both the microsampling device (red) and plasma (blue) is visualized in a density plot. The median CV is highlighted with a dashed line for each subset. (C) The quantitative difference between neat plasma and the microsampling device is small for the vast majority of the quantified peptides, with the highest histogram bin being centered above 0. (D) The quantitative difference at the protein level between the microsampling device and neat plasma is represented by a bar for each donor. The most significant difference is observed in the two least abundant proteins (APOF and LPA) and FGB.
Anal. Chem. 2025, 97, 25, 12953–12962: Figure 3. (A) A scatterplot of all peptides quantified in the nine healthy donors, showing a strong correlation. The peptides are plotted with the normalized log2(ratio to standard) from the microsampling device on the y-axis and the normalized log2(ratio to standard) from the plasma on the x-axis. (B) The precision of the measurements in both the microsampling device (red) and plasma (blue) is visualized in a density plot. The median CV is highlighted with a dashed line for each subset. (C) The quantitative difference between neat plasma and the microsampling device is small for the vast majority of the quantified peptides, with the highest histogram bin being centered above 0. (D) The quantitative difference at the protein level between the microsampling device and neat plasma is represented by a bar for each donor. The most significant difference is observed in the two least abundant proteins (APOF and LPA) and FGB.
To further explore the reason for the outlier cluster, we sought to assess whether this is caused by the quick preparation in an EDTA-free environment of the DPS compared to the conventional centrifugation-based workflow or if it is caused by the device itself. To do this assessment, the Capitainer Plasma card was loaded with plasma rather than blood. These samples were prepared as before but were analyzed with label-free SRM-based quantification. The data were normalized to albumin, which was assigned as a global standard in Skyline. The results can be seen in Figure 4, which shows that there is both a decrease and a higher variance in the FGB peptide levels of the plasma loaded in the microsampling device compared to the neat plasma (Figure 4A). The same quantitative offset observed for FGB in the capillary blood and the venous blood prepared in the microsampling device compared to neat plasma (Figure 2B and Figure 3A) is observed in the neat plasma processed through the device, while the other proteins still show a good quantitative correlation with the neat plasma sample (Figure 4B). This indicates that processing of the sample in the device specifically impacts FGB.
 Anal. Chem. 2025, 97, 25, 12953–12962: Figure 4. (A) By loading plasma onto the microsampling device, an increase in variance and a decrease in peptide amounts for FGB are observed. (B) Despite the impact of the microsampling device on FGB, the peptides originating from other proteins show a good quantitative correlation with neat plasma, highlighting that the observed behavior is specific for FGB. The log2(ratio to global standard) from the microsampling device is presented on the y-axis, and the log2(ratio to global standard) from the neat plasma is presented on the x-axis.
Anal. Chem. 2025, 97, 25, 12953–12962: Figure 4. (A) By loading plasma onto the microsampling device, an increase in variance and a decrease in peptide amounts for FGB are observed. (B) Despite the impact of the microsampling device on FGB, the peptides originating from other proteins show a good quantitative correlation with neat plasma, highlighting that the observed behavior is specific for FGB. The log2(ratio to global standard) from the microsampling device is presented on the y-axis, and the log2(ratio to global standard) from the neat plasma is presented on the x-axis.
Long-Term Stability Evaluation
To assess the long-term stability of the quantified proteins in the microsampling device while stored at RT, five replicate microsample cards were prepared from venous blood and moved to −20 °C at different times, spanning 232 days. Once all samples had been moved to storage at −20 °C, the samples were prepared for LC–MS/MS analysis, and all peptide quantities were normalized to the predetermined plasma level of albumin in the particular sample (940 pmol/μL). As it had been determined that the FGB levels could not be reliably quantified using the microsampling device, FGB was excluded from the analysis. For the remaining proteins, a reproducible quantification was obtained across the entire experiment, as visualized at a peptide level in Figure 5A. Figure 5A also highlights the dynamic range that is quantified in this experiment, stretching from roughly 1300 pmol/μL to 10 fmol/μL. It is worthwhile to note the discrepancy in the amount provided by the two peptides from HPX. The peptide YYCFQGNQFLR resides in a repeat sequence and, therefore, provides a higher molar amount, while SWPAVGNCSSALR spans two repeats and thus only appears once in the protein sequence. The quantitative stability at the protein level is illustrated in Figure 5B,C, showcasing high stability in most proteins. It is, however, noteworthy that APOC1 and APOB appear to degrade over time, and the most impactful changes happen in the first 50 days. In Figures S3–S22, figures for all proteins at the peptide level can be observed.
 Anal. Chem. 2025, 97, 25, 12953–12962: Figure 5. (A) The results obtained for all peptides across the long-term stability experiment were grouped by protein and arranged in the order of abundance. (B) The stability is illustrated at the protein level for the duration of the experiment. (C) The change from the initial quantities measured for each protein is represented as the percentage change compared to day 1. Due to the inconsistent quantification of FGB, it has been excluded from all three figures.
Anal. Chem. 2025, 97, 25, 12953–12962: Figure 5. (A) The results obtained for all peptides across the long-term stability experiment were grouped by protein and arranged in the order of abundance. (B) The stability is illustrated at the protein level for the duration of the experiment. (C) The change from the initial quantities measured for each protein is represented as the percentage change compared to day 1. Due to the inconsistent quantification of FGB, it has been excluded from all three figures.
Conclusion
We conducted a thorough quantitative evaluation and benchmarking of a novel microsampling device for readily preparing DPS from finger pricks. Our findings show that the samples prepared using the microsampling device showed high precision, with the majority of the peptides exhibiting a CV below 10% and a median CV of 1.6% in a cohort of nine healthy donors based on a biomarker panel consisting of 19 proteins (57 peptides). There is a noticeable variation in the amount sampled when using the microsampling device. Yet this variance is easily overcome by normalizing the peptide quantities to ALB, and the volumetric sampling still serves as a valuable way of ensuring that the spiked-in standards are added in an appropriate amount. Our data also show a high correlation between the quantitative results obtained in plasma and those obtained using the microsampling device, indicating that the quantitative results can be translated between the two sample types. However, certain targets, like FGB, are directly impacted by the sample collection method. Therefore, it will always be essential to validate the targets in a target-specific way to ensure that the sampling method does not impact the analytes of interest. The results also show that for quite a few of the targets, the dried format helps stabilize the targets and that the samples can even be stored for seven months at RT without drastically impacting the quantitative results. However, as with all sample collection, it is advised to ensure that a standardized method of handling the samples after collection is well established. Our findings suggest that the microsampling device for preparing DPS straight from capillary blood is very promising and has a high potential to drastically increase the samples that can be collected in a clinical trial without a significant patient burden and thereby provide a more detailed characterization of the effects of drugs evaluated in clinical trials at a patient-centric level.