Menu
More information
WebinarsAbout usContact usTerms of use
LabRulez s.r.o. All rights reserved. Content available under a CC BY-SA 4.0 Attribution-ShareAlike
Scientific article | Science and research
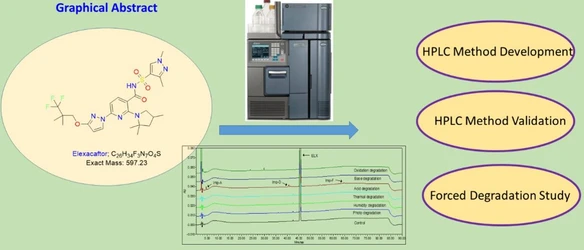
Quality-by-Design-Driven RP-HPLC Method Development and Validation for Impurity Analysis of Elexacaftor, a Cystic Fibrosis Drug, with LC-MS/MS-Based Degradant Identification
A validated QbD-based RP-HPLC method enables robust, stability-indicating impurity analysis of Elexacaftor, supporting process development and quality control in drug manufacturing.
Mo, 22.12.2025
LabRulez


Interview | Video
Natural Product Discovery through Liquid Extraction Based Ambient ionization Mass Spectrometry
Interview with PhD researcher Jess on using ambient ionization mass spectrometry to screen and prioritize novel metabolites, reduce solvent use, and streamline natural product discovery before chromatography.
Fr, 19.12.2025
Organomation


Article | Science and research
The butterfly – a symbol of hope for oncology patients – chosen as the emblem of Taveren Therapeutics, a new biotech company emerging from IOCB Prague
IOCB Prague is co-founding Taveren Therapeutics, a new biotech spin-off developing a novel cancer treatment strategy based on reactivating the body’s natural tumor suppressors.
Fr, 19.12.2025
Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences


Article | Product
Automated Device Shutdown After Sequence Completion in Clarity Chromatography Software
Learn how shutdown methods in Clarity Sequence Options enable automatic post-sequence actions such as cooling devices, switching off lamps, or sending shutdown commands after the last run.
Tu, 16.12.2025
DataApex


Article | Science and research
Beyond Blood: Sheffield Teaching Hospitals NHS Foundation Trust Develops HRAM-Powered Method for Post-Mortem Toxicology Analysis
Sheffield researchers validated Thermo Scientific Tox Explorer for LC-MS/MS drug screening in post-mortem vitreous humor, confirming its sensitivity, stability and forensic value.
Tu, 16.12.2025
Thermo Fisher Scientific

Scientific article | Science and research
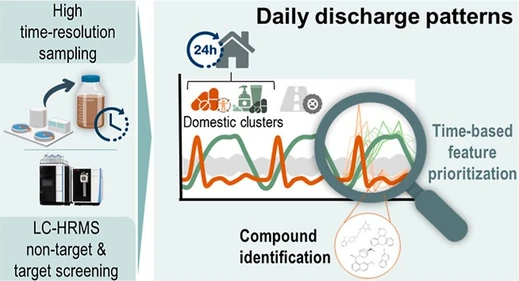
Exploring Domestic Discharge Patterns in Wastewater through LC-HRMS Screening and Temporal Clustering
LC-HRMS screening combined with temporal clustering identified daily domestic discharge patterns in wastewater, enabling improved interpretation of population chemical exposure.
Mo, 15.12.2025
LabRulez

More information
WebinarsAbout usContact usTerms of use
LabRulez s.r.o. All rights reserved. Content available under a CC BY-SA 4.0 Attribution-ShareAlike